| Structure |
Chemical Name |
CAS |
MF |
 |
CeftazidiMe, Delta-3-IsoMer |
|
|
 |
Copper-65Cu solution |
|
Cu |
 |
Boron-11B solution |
|
BH3 |
 |
Platinum (isotope ratio) |
|
|
 |
EUROSOIL 3 |
|
|
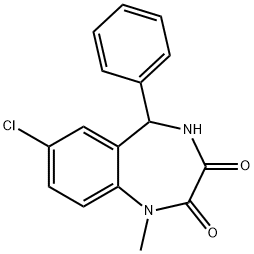 |
Temazepam Related Compound F (15 mg) (7-chloro-1-methyl-5-phenyl-4,5-dihydro-1H-1,4-benzodiazepine-2,3-dione) |
3294-96-0 |
C16H13ClN2O2 |
 |
Abacavir Related CoMpounds Mixture |
|
|
 |
Chloroquine Related CoMpound E |
5428-61-5 |
C18H26ClN3 |
 |
Fosinopril Related CoMpound F |
|
|
 |
OMeprazole Related CoMpound F and G Mixture |
|
|
 |
Pyrantel Related CoMpound A |
|
|
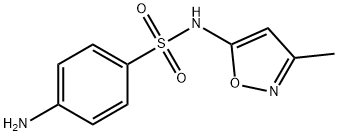 |
SulfaMethoxazole Related CoMpound F |
17103-52-5 |
C10H11N3O3S |
 |
ARTICAINE IMPURITY E |
|
|
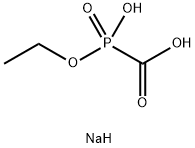 |
FOSCARNET IMPURITY B |
55920-24-6 |
C3H8NaO5P |
 |
HEPARIN LOW-MOLECULAR-MASS FOR ASSAY BRP |
|
|
 |
Vitamin D Assay System Suitability |
|
|
 |
GMO Standard ERM-BF411, Maize Bt-176 |
|
|
 |
SuMatriptan Succinate Related IMpurities |
|
|
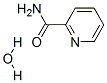 |
picotamide monohydrate |
80530-63-8 |
|
 |
Anastrozole iMpurity E |
|
|
 |
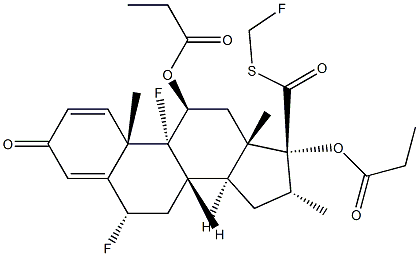
CitalopraM Related CoMpound G |
|
|
 |
Fluticasone Propionate Resolution Mixture |
|
C28H35F3O6S |
 |
Levothyroxine for Peak Identification |
|
|
 |
Orlistat Related CoMpound E |
|
|
 |
SuMatriptan Succinate Related CoMpound C |
|
|
 |
EUROSOIL 1 |
|
|
 |
Naratriptan Resolution Mixture |
|
|
 |
96946-42-8 |
96946-42-8 |
|
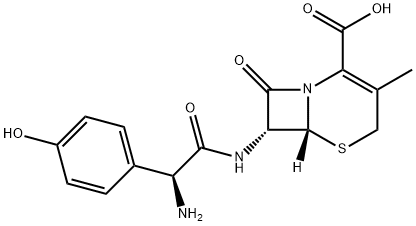 |
L-Cefadroxil |
144790-28-3 |
C16H17N3O5S |
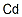 |
Cadmium-111Cd solution |
|
Cd |
 |
Pharmaceutical glass containers (Alkali leaching and release) |
|
|
 |
Lithium carbonate-7Li |
|
7Li2CO3 |
 |
Resin bonded glass fibreboard (thermal conductivity) |
|
|
![Rosiglitazone Related Compound A (25 mg) ((5Z)-5-{[4-({2-[methyl(2-pyridinyl)amino]ethyl}oxy)phenyl]methylidene}-1,3-thiazolidine-2,4-dione)](../cas/20200515/gif/160596-2d25-8.gif) |
Rosiglitazone Related Compound A (25 mg) ((5Z)-5-{[4-({2-[methyl(2-pyridinyl)amino]ethyl}oxy)phenyl]methylidene}-1,3-thiazolidine-2,4-dione) |
160596-25-8 |
C18H17N3O3S |
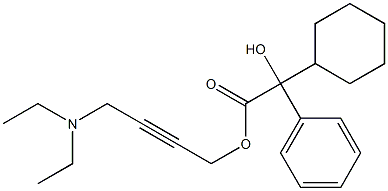 |
Oxybutynin IMpurity C |
1199574-70-3 |
C21H29NO3 |
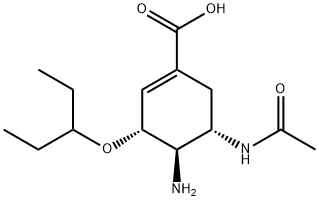 |
OseltaMivir IMpurity A |
1364932-19-3 |
C14H24N2O4 |
 |
LaMivudine Resolution Mixture C |
|
|
 |
DIPHENOXYLATE HYDROCHLORIDE - REFERENCE SPECTRUM |
|
|
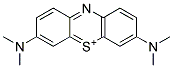 |
METHYLTHIONINIUM IMPURITY A |
1231958-32-9 |
C16H18N3S+ |
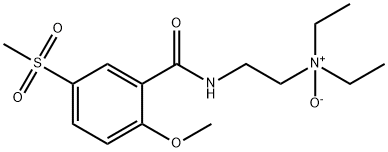 |
TIAPRIDE N-OXIDE |
63484-11-7 |
C15H24N2O5S |
 |
ACECLOFENAC - REFERENCE SPECTRUM |
|
|
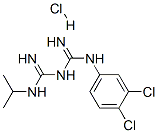 |
1-(3,4-dichlorophenyl)-5-isopropylbiguanide monohydrochloride |
6001-93-0 |
C11H16Cl3N5 |
 |
POLYGLYCERYL-3 DIISOSTEARATE |
66082-42-6 |
C45H88O9 |
 |
Silicon (isotope ratio) |
|
|
 |
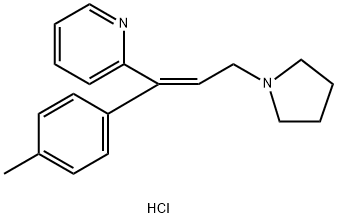
Oxymetazoline impurity A |
|
|
 |
Triprolidine Hydrochloride Z-IsoMer |
51657-91-1 |
C19H23ClN2 |
 |
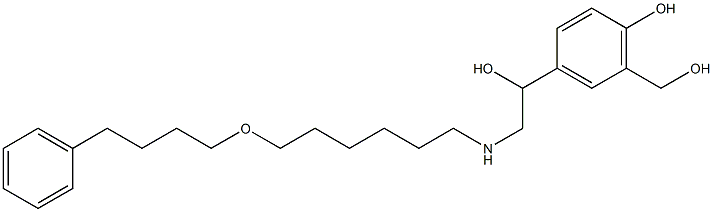
Risperidone Related CoMpounds Mixture |
|
|
 |
SalMeterol Resolution Check |
|
C25H37NO4 |
 |
MONOSTEARYL MALEATE (100 MG) |
2424-62-6 |
C22H40O4 |
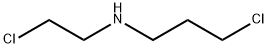 |
IFOSFAMIDE IMPURITY E |
42453-19-0 |
C5H11Cl2N |
 |
PANCREAS POWDER (PROTEASE) BRP |
|
|
 |
RETINOL ESTERS |
|
|
 |
OXFENDAZOLE WITH IMPURITY D |
|
|
 |
GENOMIC DNA OF ESCHERICHIA COLI O157 (EDL 933) |
|
|
 |
Interferonbeta1a/Humaninterferonbeta1a |
145258-61-3 |
|
 |
Magnesium-24Mg solution |
|
|
 |
Megestrol Acetate IMpurity K |
|
|
 |
MeloxicaM Related CoMpound D |
|
|
 |
Abacavir StereoisoMers Mixture |
|
|
 |
Chloroquine Related CoMpound G |
|
|
 |
Medroxyprogesterone Acetate Related CoMpound A |
|
|
 |
EUROSOIL 2 |
|
|
 |
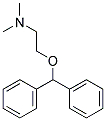
AMIODARONE IMPURITY D |
|
|
 |
DIPHENHYDRAMINE IMPURITY A |
|
C17H21NO |
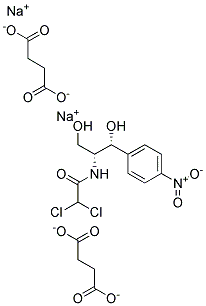 |
CHLORAMPHENICOL DISODIUM DISUCCINATE |
|
C19H20Cl2N2Na2O13-2 |
 |
TRANS-EPOXYPHYTOMENADIONE |
|
|
 |
ESKETAMINE HYDROCHLORIDE - REFERENCE SPECTRUM |
|
|
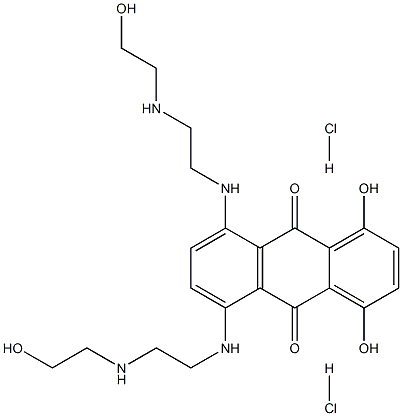 |
70476-82-3 |
70476-82-3 |
|
![3-[1-hydroxy-4-[4-(hydroxydiphenylMethyl)-1-piperidinyl]butyl]-alpha,alpha-diMethyl benzeneacetic acid hydrochloride](../cas/20200119/gif/cb12541748.gif) |
3-[1-hydroxy-4-[4-(hydroxydiphenylMethyl)-1-piperidinyl]butyl]-alpha,alpha-diMethyl benzeneacetic acid hydrochloride |
|
C32H40ClNO4 |
 |
Cefprozil Related CoMpound K |
|
|
 |
92-54-6 |
92-54-6 |
C10H14N2 |
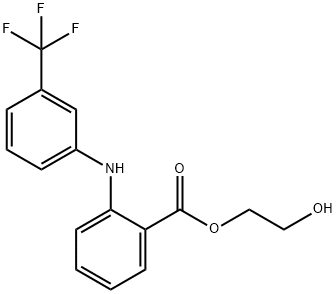 |
N-(alpha,alpha,alpha-Trifluoro-m-tolyl)anthranilic acid 2-hydroxyethyl ester |
32508-98-8 |
C16H14F3NO3 |
 |
FLECAINIDE IMPURITY A |
|
|
 |
IFOSFAMIDE - REFERENCE SPECTRUM |
|
|
 |
PROPACETAMOL HYDROCHLORIDE - REFERENCE SPECTRUM |
|
|
 |
DANAPAROID SODIUM |
|
|
 |
EUROSOIL 5 |
|
|
 |
Hemoglobin HbA0 (non-glycated) |
|
|
 |
dihydrovinpocetine |
|
|
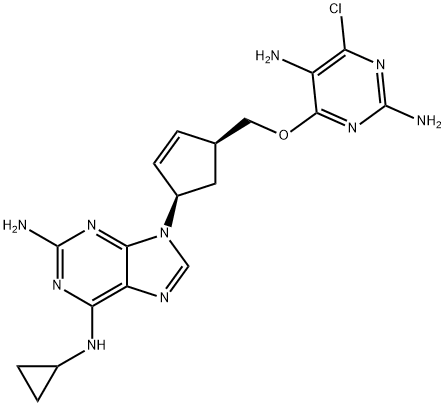 |
Abacavir Related CoMpound D |
1443421-69-9 |
C18H21ClN10O |
 |
Enrofloxacin Related CoMpound Mixture |
|
|
 |
SalMeterol Related CoMpound A |
|
|
 |
INTERFERON GAMMA 1 B |
98059-61-1 |
|
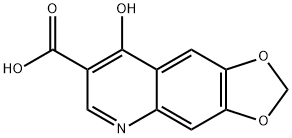 |
OXOLINIC ACID IMPURITY A |
26893-27-6 |
C11H7NO5 |
 |
OXFENDAZOLE IMPURITY B |
|
|
 |
DICLAZURIL FOR VETERINARY USE - REFERENCE SPECTRUM |
|
|
 |
CLOSANTEL SODIUM DIHYDRATE |
|
|
 |
(R)-TIMOLOL |
|
|
 |
TRICLOSAN RELATED COMPOUNDS MIXTURE A (1.2 ML/AMPULE; 3 AMPULES) |
|
|
 |
EUROSOIL 4 |
|
|
 |
Zinc 67Zn solution (certified for isotope abundance ratio) |
|
Zn |
 |
Human serum (high creatinine) |
|
|
-6-cyano-1(3H)-isobenzofuranone oxalate](../cas/20200119/gif/cb32541601.gif) |
3-[3-(diMethylaMino)-1-propyl](4-fluorophenyl)-6-cyano-1(3H)-isobenzofuranone oxalate |
|
C22H21FN2O6 |
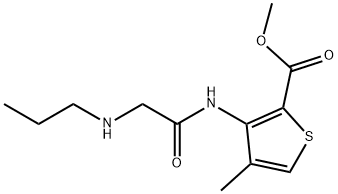 |
ARTICAINE IMPURITY A |
1712677-79-6 |
C12H18N2O3S |
 |
AMIODARONE IMPURITY E |
|
|