Cytoskeleton refers to the protein fiber network found in eukaryotic cells. It was found relatively later, mainly because that the general sample preparation of electron microscopy was performed under low temperature (0-4 °C) for cell fixation while the cytoskeleton will undergo depolymerization at low temperatures. Until the 1960s, after the use of glutaraldehyde for fixation at room temperature, people come to realize the objective existence of the cytoskeleton.
The cytoskeleton not only play important roles in maintaining the cell shape, tolerating the external force and maintaining the order in terms of internal structure of cells, but also participate into many important life processes (FIG. 1), for example: during the process of cell division, the cytoskeleton will hold the chromosome for segregation; during the cellular material transport, all types of vesicles and organelles can be directed along cytoskeleton; in muscle cells, cytoskeleton can from power system together with its binding proteins; the migration of leukocytes, motility of sperm, the stretching of nerve cell axons and dendritic are all related to cytoskeleton. Furthermore, in plant cells, the cytoskeleton directs the synthesis of the cell wall.
Cytoskeleton consists of microfilament, microtubules and intermediate filaments. Microfilament determines the surface characteristics of the cells, enabling the cells to move and contract. Microtubules determine the position of the membranous organelles (membrane-enclosed organelle) and as the rail of the vesicular transport. Intermediate fiber makes the cells to have tension and anti-shear forces.
Microfilaments, microtubules and intermediate filaments are located in the cytoplasm, also known as the cytoskeleton. They are all comprised of monomeric proteins which bind together via weak non-covalently bond, forming fiber-type polymer. It is easy to undergo assembly and disassembly, which is necessary to achieve its functional characteristics.
Generalized cytoskeleton also includes nucleoskeleton, nuclear lamina and extracellular matrix, forming an integrated network structure through the cell nucleus, cytoplasm and outer cell.
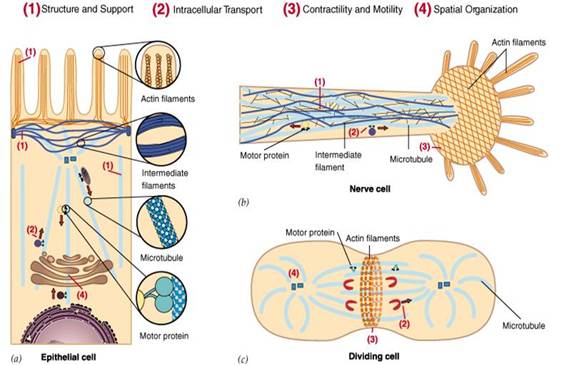
Figure1 the diagram of the major functions of the cytoskeleton
Cytoskeleton is the structural basis of cell function. Upon external stimuli or during the process of cell division and differentiation, cell will undergo related morphological changes. This is inseparable from the regulation of the Cytoskeletal dynamic change via signaling transduction. Cytoskeleton, as the dynamic protein fiber network inside eukaryotic cell, consists of the microfilaments (microfilaments, MF), tubulin (microtubules, MT) and an intermediate fiber (intermediate filaments, IF). MF is the polymer formed through actin polymerization and is widely present in the stress fibers, adhesion focal, pseudopodia and the contractile ring of the eukaryotic cells. Its structure constituent unit, globular actin (G-actin), under the action of ATP and a variety of actin-related proteins, through the continuous conversion of assembly and depolymerization with the filamentous actin (F-actin), participating into the regulation of behaviors of cell morphology, adhesion, migration, and cytokinesis. MT is formed through the polymerization of α-tubulin and β-tubulin. Under the action of microtubule-associated proteins, it assembles to form a hollow tubular structure, playing an important role in intracellular material transport and cytokinesis. MF and MT cytoskeleton are interrelated and interdependent, jointly participating into the regulation of cell behavior (Bailly M, 2002; Miranti C.K, 2002).
Cell migration is the key step during the process of malignant tumor invasion and metastasis. Cytoskeleton and its binding protein is the material basis of cell migration. Cell cytoskeleton is needed for the directional movement of cells. The microfilament cytoskeleton consisting of actions are in particular important during the above process. MF and MT not only play important role in stabilizing the cell morphology, withstanding the external stimulation and maintenance of the order of the internal structure of cells, but also participate in the regulation of cell migration, adhesion, division and intracellular signaling. Among them, the interaction effect between the cAMP-mediated signaling pathway and cytoskeleton are closely related to various cellular behaviors including cell proliferation, apoptosis, adhesion and migration. cAMP-dependent protein kinase A (PKA), as the major cAMP-dependent protein targeting protein, is involved in these processes. PKA can activate the RhoA through phosphorylation. RhoA, after activation, in addition of further activating downstream pathways, also enhances the binding capability to the Rho guanosine diphosphate dissociation inhibitor (RhoGDI,), thus inhibiting the RhoA activity from the opposite direction (Mehlen, P, 2006).
Integrin is an important class of receptor in the cell surface of both adhesion and signal transduction function. It is a kind of hetreodimer which is composed of α and β subunits through non-covalent bonds. Extracellular matrix proteins such as fibronectin, laminin and collagen are its primary ligand; secondly there are also some cell surface molecules, soluble proteins that can also binds to integrins. Through binding to the cytoskeleton, signal transduction and other kinds of protein via its extracellular domain as well as intracellular domain, integrin, like a bridge, mediates the two-way transmission of information inside and outside of the cells. The bidirectional signal transduction between integrin and cytoskeleton, (a) from the cytoskeleton to integrins, integrin can disperse in the cell surface in the absence of binding and is not connected with the actin cytoskeleton. Once binding to the ECM ligand binding, the integrin can contact with the cytoskeleton through its intracellular domain and form clusters. (B) From integrins to the cytoskeleton, integrins can have feedback regulation of cytoskeleton assembly. The integrin-mediated adhesion itself can activate Rho family of small G proteins (Mitra SK, 2005; Larsen M, 2003; Turner CE, 2000).