|
| | Flecainide Basic information |
| Product Name: | Flecainide | | Synonyms: | N-(2-Piperidinylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)benzamide;FLECAINTDE BASE;Benzamide, N-(2-piperidinylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)-;Flecaine;rac Flecainide;Flecainide-d4;N-(Piperidin-2-ylMethyl)-2,5-bis(2,2,2-trifluoroethoxy)benzaMide;-2,5-bis(2,2,2-trifluoroethoxy) | | CAS: | 54143-55-4 | | MF: | C17H20F6N2O3 | | MW: | 414.34 | | EINECS: | 685-650-9 | | Product Categories: | Aromatics;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 54143-55-4.mol |  |
| | Flecainide Chemical Properties |
| | Flecainide Usage And Synthesis |
| Description | From the chemical point, flecainide is an analog of procainamide, to which a 2.2.2-trifluoroethoxyl
group was added at C2 and C3 of the benzene ring, and a diaminoethyl side
chain is ended in the piperidine ring. | | Chemical Properties | White Crystalline Powder | | Originator | Tambocor,Kettelhack Riker,W. Germany,1982 | | Uses | Flecainide, as with other local anesthetics, is used for naturally occurring ventricular arrhythmia. | | Uses | Flecainide is an antiarrhythmic (class IC). | | Definition | ChEBI: A monocarboxylic acid amide obtained by formal condensation of the carboxy group of 2,5-bis(2,2,2-trifluoroethoxy)benzoic acid with the primary amino group of piperidin-2-ylmethylamine. An antiarrhythmic agent used (in the form of its acetate salt) to prev
nt and treat tachyarrhythmia (abnormal fast rhythm of the heart). | | Manufacturing Process | Under a nitrogen atmosphere 2-aminomethylpiperidine (0.249 mol, 28.4 g) is
treated dropwise over 25 minutes with 2,2,2-trifluoroethyl 2,5-bis(2,2,2-
trifluoroethoxy)benzoate (0.0249 mol, 10.0 g). After 3 hours 50 ml of
benzene is added to the thick mixture and stirred for about 40 hours at 45°C.
The mixture is then concentrated under vacuum with heating to remove the
volatile components. The residue solidifies after cooling, is steam distilled for
further purification and is separated by filtration and extracted into
dichloromethane. The dichloromethane solution is washed with saturated
sodium chloride solution, and the organic layer is dried over anhydrous
magnesium sulfate. The magnesium sulfate is removed by filtration and 4 ml
of 8.4 N hydrogen chloride in isopropanol is added to the dichloromethane
solution with stirring.After 2 hours the mixture is cooled to about 0°C and the crude product is
collected by filtration, washed with diethyl ether and dried in a vacuum oven.
After treatment with decolorizing charcoal and recrystallization from an
equivolume mixture of isopropanol and methanol, the product, 2,5-bis(2,2,2-
trifluoroethoxy)-N-(2-piperidylmethyl)benzamide hydrochloride has a MP of
228°C to 229°C. | | Brand name | Tambocor
(3M Pharmaceuticals). | | Therapeutic Function | Antiarrhythmic | | World Health Organization (WHO) | The membrane-stabilizing antiarrhythmic agent flecainide was
introduced into medicine in 1982. The decision to delete the indications for
patients with asymptomatic and less severe symptomatic ventricular arrhythmias
was taken on the basis of the results of a trial (CAST study) that showed a two-fold
increase in deaths in post-myocardiac patients taking flecainide compared with the
placebo group. | | Hazard | Human systemic effects. | | Clinical Use | Flecainide (Tambocor) is a fluorinated aromatic hydrocarbon
examined initially for its local anesthetic
action and subsequently found to have antiarrhythmic
effects. Flecainide inhibits the sodium channel, leading
to conduction slowing in all parts of the heart, but
most notably in the His-Purkinje system and ventricular
myocardium. It has relatively minor effects on repolarization.
Flecainide also inhibits abnormal automaticity.
Flecainide is effective in treating most types of atrial arrhythmias.
It is also used for life-threatening ventricular
arrhythmias. However, flecainide should be used with extreme
caution in any patient with structural heart disease.
Flecainide crosses the placenta, with fetal levels reaching
approximately 70% of maternal levels. In many centers,
it is the second-line drug after digoxin for therapy of fetal
arrhythmias. Because of the high incidence of proarrhythmia,
initiation of therapy or significant increases in
dosing should be performed only on inpatients. | | Side effects | Most adverse effects occur within a few days of initial
drug administration. The most frequently reported effects
are dizziness, light-headedness, faintness, unsteadiness,
visual disturbances, blurred vision (e.g., spots before
the eyes, difficulty in focusing), nausea, headache,
and dyspnea.
Worsening of heart failure and prolongation of the PR
and QRS intervals are likely to occur with flecainide, and
an increased risk of proarrhythmia has been reported. | | Synthesis | Flecainide, N-(2-piperidylmethyl)-2,5-bis-(2,2,2-trifluoroethoxy)benzamide
(18.1.14), is synthesized from 2,5-dihydroxybenzoic acid. Reacting this with trifluoroethylfluoromethylsulfonate
gives 2.2.2-trifluoroethoxylation of all three hydroxyl groups, to produce
2,2,2-trifluoroethyl ester of 2,5-bis-(2,2,2-trifluoroethoxy)benzoic acid (18.1.12).
Reacting this with 2-aminomethylpiridine gives the corresponding amide (18.1.13), which
upon reduction of the pyridine ring with hydrogen gives flecainide (18.1.14). 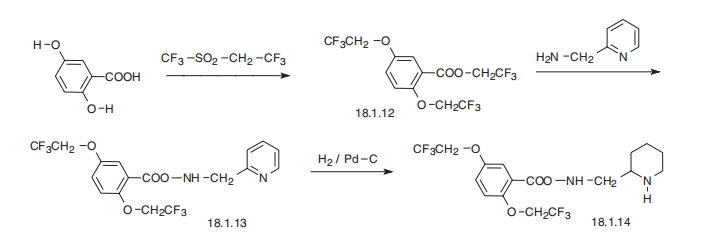
| | Drug interactions | In patients whose condition has been stabilized by flecainide,
the addition of cimetidine may reduce the rate
of flecainide’s hepatic metabolism, increasing the potential
for toxicity. Flecainide may increase digoxin concentrations
on concurrent administration. | | Precautions | Flecainide is contraindicated in patients with preexisting
second- or third-degree heart block or with bundle
branch block unless a pacemaker is present to maintain
ventricular rhythm. It should not be used in patients
with cardiogenic shock. |
| | Flecainide Preparation Products And Raw materials |
| Raw materials | Magnesium sulfate-->2-Picolylamine-->2,5-Dihydroxybenzoic acid-->2-PIPERIDYLMETHYLAMINE-->Hydrochloric acid-->2,5-Bis(2,2,2-trifluoroethoxy)benzoic acid-->2,2,2-TRIFLUOROETHYL 2,5-BIS(2,2,2-TRIFLUOROETHOXY)BENZOATE-->METHYL 2,5-BIS(2,2,2-TRIFLUOROETHOXY)BENZOATE |
|



