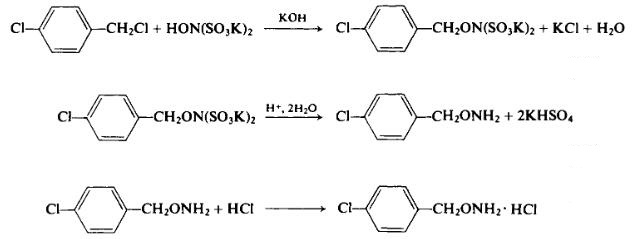
| Preparation | To a solution of 83.7 gm (1.5 moles) of potassium hydroxide in 560 ml of water is added in turn with stirring, 300 gm (0.985 mole) of dipotassium hydroxylaminedisulfonate and 161 gm (1.0 mole) of 4-chlorobenzyl chloride. The stirred mixture is heated on a steam bath for 1.75 hr. After cooling, the precipitate is filtered off and refluxed for 3 hr in 1000 ml of 1.5 Ν sulfuric acid. The reaction mixture is then cooled and made strongly alkaline with a 15% aqueous sodium hydroxide solution. The mixture is then extracted with ether repeatedly. The ether extracts are combined and evaporated to dryness. The solid residue (crude free base, 80 gm, i.e., 51.5% of theory, m.p. 35-38°C) is taken up in 1 liter of ether. Anhydrous hydrogen chloride is bubbled through this solution to precipitate the hydrochloride salt. The yield is 59 gm (31%, overall), m.p. 243-244°C (dec); the free base has b.p. 100°C/1 mm Hg. In this preparation, the hydrolysis of the alkylated dipotassium hydroxylaminedisulfonate with only 1 liter of 1.5 Ν sulfuric acid seems somewhat skimpy. We suggest that a higher level of sulfuric acid be considered at that stage of the reaction, if the alkylation has indeed gone to completion. Once the O-alkylhydroxylamine has been prepared by this reaction, it may be N-alkylated to produce an N,Ο-dialkylhydroxylamine.
The preparation of O-phenylhydroxylamine has been a synthetic challenge for some time since it is quite unstable. Using hydroxylamine-O-sulfonic acid. A small quantity of this compound has been prepared [36]. Reaction conditions probably require further study to improve the yield. Extension of the reaction to the preparation of O-alkylhydroxylamines would be of interest.
|