|
| | Gallamine triethiodide Basic information |
| Product Name: | Gallamine triethiodide | | Synonyms: | (v-phenenyltris(oxyethylene))tris(triethylammonium)iodide;(v-phenenyltris(oxyethylene))tris(triethylammoniumiodide);(v-phenenyltris(oxyethylene))tris(triethylammoniumtriiodide);3.697r.p.;benzcurineiodide;Ethanaminium,2,2',2''-[1,2,3-benzenetriyltris(oxy)]tris[N,N,N-triethyl-, iodide (1:3);f2559;flacedil | | CAS: | 65-29-2 | | MF: | C30H60I3N3O3 | | MW: | 891.53 | | EINECS: | 200-605-1 | | Product Categories: | Methotrexate;Organics;Cholinergics;Antagonists;Neurotransmitters | | Mol File: | 65-29-2.mol |  |
| | Gallamine triethiodide Chemical Properties |
| Melting point | 235 °C (dec.) (lit.) | | density | 1.4288 (estimate) | | storage temp. | 2-8°C | | solubility | H2O: 100 mg/mL | | form | powder | | color | Crystals from Me2CO (aq) | | Merck | 13,4364 | | InChIKey | REEUVFCVXKWOFE-UHFFFAOYSA-K | | SMILES | C1(OCC[N+](CC)(CC)CC)=C(C=CC=C1OCC[N+](CC)(CC)CC)OCC[N+](CC)(CC)CC.[I-].[I-].[I-] |
| | Gallamine triethiodide Usage And Synthesis |
| Chemical Properties | White Solid | | Originator | Flaxedil,Davis and Geck,US,1951 | | Uses | Muscle relaxant;M2 antagonist allosteric | | Uses | Gallamine Triethiodide is used as a neuromuscular blocking agent, paralyzing locally during anesthetization. | | Uses | Gallamine triethiodide has been used:
- as a relaxant for measuring spinal trigeminal nucleus recordings from single neurons.
- as an antagonist in neuroblastoma cells as M2 receptor
- to reduce eye movement during retinal surgery in rat
| | Manufacturing Process | 12.6 grams of pyrogallol are dissolved in 100 cc of hot toluene. 14 grams of
sodamide (85%)are added to the solution at about 100°C in 5 portions over a
period of 15 minutes, with agitation. There are then added with agitation,
over a period of 30 minutes, 100 cc of a toluene solution containing 474
grams of diethylaminochlorethane per liter of toluene.
The mixture is then heated for 1 hour, the toluene being refluxed, whereafter
it is left to cool, 50 cc of water are added and, after decanting, the solution is again washed with two quantities of 50 cc of water. The toluene solution is
dried over potassium carbonate and distilled in vacuo. There is thus obtained
28 grams of 1.2.3-tri-(β-diethylaminoethoxy)benzene, boiling at 206°C under
1 mm pressure.
20 grams of 1.2.3-tri-(β-diethylaminoethoxy)-benzene is heated for 5 hours
under reflux on the water bath with 30 grams of ethyl iodide. The hot mixture
is dissolved in 50 cc of water, filtered after addition of 2 grams of decolorizing
black, evaporated to dryness on the water bath and recrystallized from 120 cc
of alcohol. The product can be further recrystallized in mixtures of acetone
and water.
The triethiodide of 1.2.3-tri-(β-diethylaminoethoxy)-benzene is thus obtained
as white crystals which, after drying, have a rather indefinite melting point at
about 152° to 153°C, (Maquenne block). | | Therapeutic Function | Muscle relaxant | | General Description | Gallamine triethiodide,[v-phenenyl-tris(oxyethylene)]tris[triethylammonium] triiodide(Flaxedil), is a skeletal muscle relaxant that works byblocking neuromuscular transmission in a manner similar tothat of d-tubocurarine (i.e., a nondepolarizing blockingagent). It does have some differences, however. It has astrong vagolytic effect and a persistent decrease in neuromuscularfunction after successive doses that cannot be overcome by cholinesterase inhibitors. Gallamine triethiodidealso has muscarinic antagonistic properties and bindswith greater affinity to the M2 receptors than to the M1 receptor.This latter characteristic may cause its strongvagolytic action. | | Biochem/physiol Actions | Gallamine triethiodide has anti-muscarinic effect. It is a competitive antagonist for the muscarinic receptor. Gallamine is regarded as neuromuscular blocking agent. | | Clinical Use | Gallamine Triethiodide is contraindicated in patients with myastheniagravis, and one should remember that its action is cumulative,as with curare. The antidote for gallamine triethiodideis neostigmine. | | Safety Profile | Poison by ingestion, subcutaneous, intravenous, parenteral, intraduodenal, intraperitoneal, and intramuscular routes. Whenheated to decomposition it emits very toxic fumes of NH3, NOx, and Ií. | | Synthesis | Gallamine, 1,2,3-tris-(2-triethylaminoethoxy)benzene triiodide, is
synthesized from pyrogallol, the hydroxyl groups of which are esterified by 2-diethylaminoethylchloride
in the presence of sodium amide. The resulting 1,2,3-tris-(2-triethylaminoethoxy)
benzene is further alkylated at all three nitrogen atoms by
ethyliodide, giving gallamine. 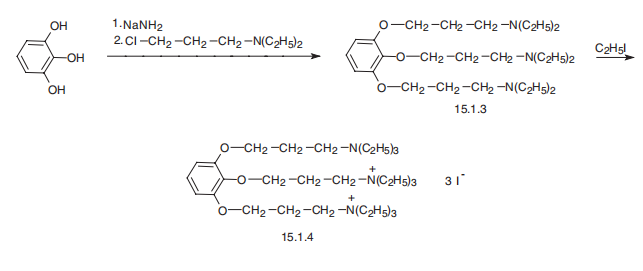
|
| | Gallamine triethiodide Preparation Products And Raw materials |
|



