|
| | CYCLOHEXYL ISOCYANIDE Basic information |
| Product Name: | CYCLOHEXYL ISOCYANIDE | | Synonyms: | Cyclohexane, isocyano-;Cyclohexaneisonitrile;BIO-FARMA BF001328;ISOCYANOCYCLOHEXANE;HANSA ISN-0519;CYCLOHEXYL ISOCYANIDE;CYCLOHEXYL ISONITRILE;Cyclohexyl #niso-cyanide | | CAS: | 931-53-3 | | MF: | C7H11N | | MW: | 109.17 | | EINECS: | 213-238-7 | | Product Categories: | ISONITRITE | | Mol File: | 931-53-3.mol |  |
| | CYCLOHEXYL ISOCYANIDE Chemical Properties |
| Melting point | 6.45°C | | Boiling point | 173-176 °C | | density | 0.878 g/mL at 25 °C(lit.) | | refractive index | n20/D 1.45(lit.) | | Fp | 170 °F | | storage temp. | Store at +2°C to +8°C. | | form | Liquid | | color | Clear colorless to slightly brown | | BRN | 3662332 | | Stability: | Stable. Incompatible with strong acids, strong bases, strong reducing agents, strong oxidizing agents. Flammable. | | CAS DataBase Reference | 931-53-3(CAS DataBase Reference) |
| | CYCLOHEXYL ISOCYANIDE Usage And Synthesis |
| Chemical Properties | colourless liquid | | Uses | Cyclohexyl Isocyanide can be used as novel arginase inhibitors to treat diseases. | | Preparation | CAUTION: Use a well-ventilated hood and take all precautions before using phosgene.
To a flask equipped as in Preparation 3-1 is added 1.27 kg (10.0 moles) of N-cyclohexylformamide, 3.20 liters of triethylamine, and 4.50 liters of methylene chloride. The solution is stirred while phosgene is rapidly added (300-400 gm/hr) to cause vigorous refluxing. Refluxing ceases after 1.04 kg (10.2 moles) of phosgene have been added and the addition is stopped. The reaction mixture is cooled to 22-25°C, 400 gm (23.5 moles) of ammonia gas added over a period of 1-2 hr, the mixture filtered then concentrated under reduced pressure, and the residue distilled to afford 955 gm (88%), b.p. 67-72°C (14 mm Hg).
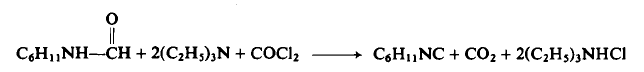
| | Definition | ChEBI: Cyclohexyl isocyanide is an isocyanide having a cyclohexyl group attached to nitrogen. It is a conjugate base of a cyclohexyl isocyanide(1+). | | General Description | Cyclohexyl isocyanide reacts with dimethyl acetylenedicarboxylate to give a mixture of cyclopenta[b]pyridine derivatives, azaspirononatriene derivative and the azabicyclononatriene. It reacts with dialkyl acetylenedicarboxylates to form 1:1 intermediate which on facile addition to 1-benzylisatin and tryptantrin yields highly functionalized novel unsaturated γ-spiroiminolactones. |
| | CYCLOHEXYL ISOCYANIDE Preparation Products And Raw materials |
|