|
| | N,O-Bis(trimethylsilyl)acetamide Chemical Properties |
| | N,O-Bis(trimethylsilyl)acetamide Usage And Synthesis |
| Description | Clear to yellowish clear liquid liquid which is soluble in many nonpolar and polar aprotic solvents.
The trimethylsilyl group is a frequently utilized monofunctional blocking group for protic organic and inorganic materials. As it eliminated during the silylation is neutral acetamide, N,O-Bis(trimethylsilyl)acetamide can be preferably used for substrates which are acid- or base-sensitive.
N,O-bis(trimethylsilyl)-acetamide is an effective silylating agent. It is used in the pharmaceutical and in the chemical industry. Typically the silylating reaction with BSA results in neutral by-products only.
| | Uses |
- Used for blocking and protection of hydroxyl groups in natural products, e.g. amino acids, carbohydrates;
- Used for blocking and protection of functional groups in organic intermediates;
- Used for formation of derivatives of H-acid compounds for analysis;
The silylated derivatives are volatile and can therefore be determined by gas chromatography. Deblocking is preferably carried out by means of hydrolysis, giving high yields. In some cases, thermal elimination of the trimethylsilyl group is possible.
| | Physical properties | bp 71–73°C/35 mmHg. | | Uses | Powerful silylation reagent for a wide range of functional groups under mild conditions | | Uses | N,O-bis(trimethylsilyl)-acetamide (BSA) is frequently used with TMCS as a catalyst to modify a variety of functional groups including carboxyl groups. In one novel application, BSA was used to measure carboxypeptidase activity by forming derivatives which could be determined by gas chromatography.BSA has been used to prepare derivatives of steroids and glucocorticoids. | | Uses | N,O-Bis(trimethylsilyl)acetamide is a powerful silylating agent; reacts with a wide range of functional
groups.
BSA was used to
trimethylsilylate the hydroxyl group of 12 selectively to form
in almost quantitative yield (eq 32).The presence of catalytic
amounts (~0.02 equiv) of tetrabutylammonium fluoride (TBAF)
significantly promoted the silylation of alcohols under mild conditions
with high chemoselectivity, i.e., TBAF plays a role as a
smooth silyl transfer catalyst from nitrogen to the hydroxyl group.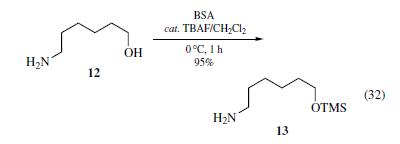
| | Preparation | made by the reaction of acetamide with
a large excess of chlorotrimethylsilane in the presence of
triethylamine. | | General Description | N,O-Bis(trimethylsilyl)acetamide is an excellent silylation reagent. For some sterically hindered primary or secondary nitro compounds, N,O-bis(trimethylsilyl)acetamide and BSTFA achieve better results than normal silylation conditions. | | Purification Methods | Fractionate it through a spinning band column and collect liquid b 71-73o/35mm, and not higher because the main impurity MeCONHSiMe3 distils at b 105-107o/35mm. It is used for derivatising alcohols and sugars [Klebe et al. J Am Chem Soc 88 3390 1966, see Matsuo et al. Carbohydr Res 241 209 1993, Johnson Carbohydr Res 237 313 1992]. Itis FLAMMABLE and TOXIC. |
| | N,O-Bis(trimethylsilyl)acetamide Preparation Products And Raw materials |
|



