|
| | Vilazodone Hydrochloride Basic information |
| Product Name: | Vilazodone Hydrochloride | | Synonyms: | 5-[4-[4-(5-Cyanoindol-3-yl)butyl]piperazin-1-yl]benzofuran-2-carboxamide hydrochloride;Vilazodone hydrochloride;5-[4-[4-(5-Cyanoindol-3-yl)butyl]piperazin-1-yl]benzofuran-2-carboxaMide hydrochloride、Vilazodone hydrochloride;EMD 68843;SB 659746A;CS-796;5-[4-[4-(5-Cyano-1H-indol-3-yl)butyl]-1-piperazinyl]-2-benzofurancarboxamide hydrochloride;VILAZODONE; EMD 68843; SB 659746A | | CAS: | 163521-08-2 | | MF: | C26H28ClN5O2 | | MW: | 477.98582 | | EINECS: | 695-883-8 | | Product Categories: | API;Inhibitors | | Mol File: | 163521-08-2.mol |  |
| | Vilazodone Hydrochloride Chemical Properties |
| Melting point | 279°C(lit.) | | Fp | 9℃ | | storage temp. | Inert atmosphere,Store in freezer, under -20°C | | solubility | DMSO: soluble20mg/mL, clear | | form | powder | | color | white to beige | | Stability: | Hygroscopic |
| | Vilazodone Hydrochloride Usage And Synthesis |
| Description | Vilazodone is a selective serotonin reuptake inhibitor (SSRI) and a partial agonist of the serotonin (5-HT) receptor subtype 5-HT1A (IC50s = 0.2 and 0.5 nM, respectively). It increases extracellular 5-HT in the rat ventral hippocampus and frontal cortex when administered intraperitoneally at doses of 1 and 3 mg/kg. Vilazodone (1 mg/kg, i.p.) decreases immobility in the forced swim test in both rats and mice. Formulations containing vilazodone have been used in the treatment of depression. | | Uses | Vilazodone Hydrochloride, is the salt form of Vilazodone (V265000), which is a combined serotonin specific reuptake inhibitor (SSRI) and 5-HT1A receptor partial agonist currently under clinical evaluation for the treatment of major depression. Antidepressant. | | Definition | ChEBI: A hydrochloride obtained by reaction of vilazodone with one equivalent of hydrochloric acid. Used for the treatment of major depressive disorder. | | Clinical Use | Althoughseveral synthetic approaches have beenreported,a process-scale synthesis of vilazodone consists of the union of an indole-containing butyl tosylate 284 with a benzofuranyl piperazine whose synthesis is described in the scheme below. Piperazine 280 arises from a Buchwald coupling of commercially available
benzofuranyl bromide 279 with piperazine through the use of a
unique catalyst system employing the DavePhos ligand. This
single coupling step, which has been executed on multigram scale
in 70% yield,210 circumvented the need for any protecting group
chemistry for either the primary amide within 279 or the piperazine
amine functionality. For the preparation of the key indole
subunit, Friedel-Crafts acylation of commercially available 5-cyanoindole
(281) proceeded in good yield at the 3-position of the indole
with 4-chlorobutanoyl chloride in 82% yield. Treatment of the
resulting chloroketone with sodium borohydride in refluxing
isopropanol converted 282 to the corresponding terminal alcohol
283. Tosylation of this alcohol was followed by displacement with
piperazine 280 to give vilazodone hydrochloride (XXV) after
acidification. | | Synthesis | Vilazodone hydrochloride is a combined serotonin reuptake
inhibitor (SSRI) and 5-HT1A receptor partial agonist marketed under
the trade name Viibryd.202 Viibryd was developed by Merck
KGaA (Germany) and approved for the treatment of depression
by the U.S. FDA on January 21st, 2011. Vilazodone has been shown
to be well-tolerated at higher dosage levels, specifically by not
causing significant weight gain or decreased sexual desire or function,
which are improvements over existing antidepressant
treatments. 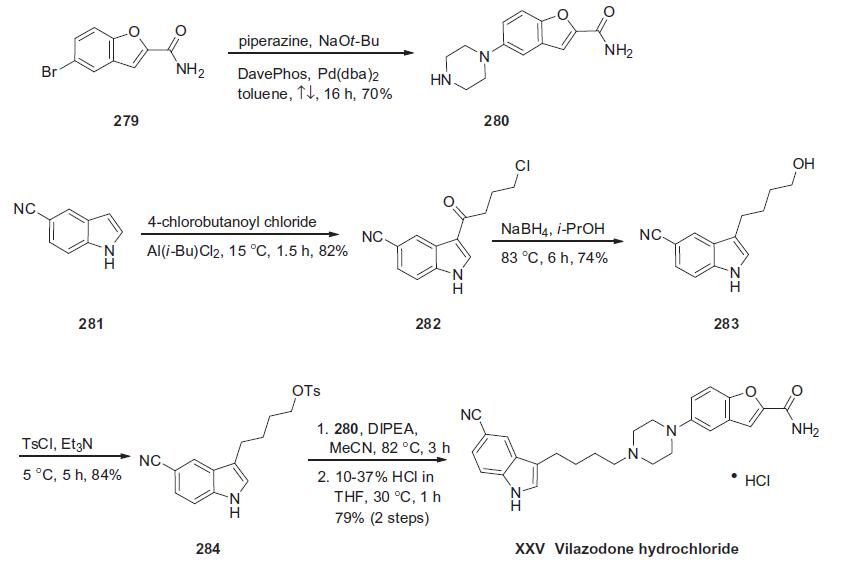
| | storage | Store at -20°C |
| | Vilazodone Hydrochloride Preparation Products And Raw materials |
|