| | Methyllithium Basic information |
| | Methyllithium Chemical Properties |
| Melting point | 70-71℃ | | Boiling point | 88℃ | | density | 0.85 g/mL at 20 °C | | vapor density | 3 (vs air) | | Fp | 5 °F | | storage temp. | 2-8°C | | form | Solution | | Specific Gravity | 0.83 | | color | Colorless to yellow | | Water Solubility | Reacts with water. | | Sensitive | Air & Moisture Sensitive | | Hydrolytic Sensitivity | 9: reacts extremely rapidly with atmospheric moisture - may be pyrophoric - glove box or sealed system required | | BRN | 3587162 | | Exposure limits | ACGIH: TWA 400 ppm; STEL 500 ppm
OSHA: TWA 400 ppm(1200 mg/m3)
NIOSH: IDLH 1900 ppm | | CAS DataBase Reference | 917-54-4(CAS DataBase Reference) | | EPA Substance Registry System | Lithium, methyl- (917-54-4) |
| | Methyllithium Usage And Synthesis |
| Chemical Properties | Methyllithium is a colorless to yellowish solution, It is insoluble in hydrocarbon solvents although propyllithium and the higher homologues are miscible in hydrocarbons. Methyllithium is soluble in diethyl ether to about 6% by weight at room temperature (with or without lithium bromide) and is a little less soluble in tetrahydrofuran. Cooling of these solutions produces clear, colorless crystals of methyllithium etherate. Methyllithium is stable to about 200°C, at which temperature it disproportionates to dilithiomethane and methane.
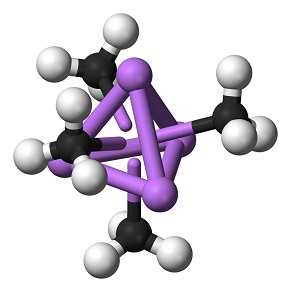
| | Uses | There are no significant industrial uses of methyllithium, but it is widely used in the laboratory in Grignard type reactions.
Methyllithium is an organolithium reagent mainly used for deprotonation and as a source of methyl anion. It is a strong base and a strong nucleophile.
| | Uses | Methyllithium lithium bromide complex solution (MeLi.LiBr) can be used as a methylating agent for the α-methylation of alkoxyaryl ketones. | | Definition | ChEBI: Methyllithium is an alkyllithium compound and a one-carbon compound. | | Preparation | Methyllithium is produced industrially by the reaction of methyl chloride or methyl bromide with lithium metal dispersion in diethyl ether. The reaction with methyl chloride yields a solution of methyllithium and a precipitate of lithium chloride which may be readily separated. When methyl bromide is used, the lithium bromide byproduct remains in solution and complexes with the methyllithium. This reduces the reactivity of the methyllithium and, in certain cases, affects its usefulness. On the other hand, the less reactive complex is more stable in diethyl ether. Methyllithium produced from methyl chloride contains only about 0.3 % lithium chloride in a 5 % solution in diethyl ether. It is more reactive than the lithium bromide complex, but decomposes at the rate of 1 % of the contained methyllithium per year (that is 5.00 % solution goes to 4.95 %) at room temperature. The decomposition by ether cleavage results in methane and ethylene formation which increases the pressure in the container on long storage. Methyllithium can be prepared easily in tetrahydrofuran but it has a half-life of only about 2 days at room temperature due to solvent cleavage. | | Application | Methyllithium solution (1.6M in diethyl ether) has been used for the asymmetric allylic alkylation (AAA) of allylic electrophiles in the presence of a chiral copper catalyst. This protocol leads to the C-C bond formation of tertiary and quaternary stereogenic centers with high enantioselectivity. It can also be used for the synthesis of (?)-salsolidine by reacting with 6,7-dimethoxy-3,4-dihydroisoquinoline in the presence of (?)-sparteine as a chiral ligand. | | Hazard | Flammable, dangerous fire and explosionrisk, self-ignites in air. |
| | Methyllithium Preparation Products And Raw materials |
|



