|
| Product Name: | Olsalazine sodium | | Synonyms: | 3,3’-azobis[6-hydroxy-benzoicacidisodiumsalt;5,5'-Azobis(2-hydroxybenzoic acid sodium) salt;5,5'-Azobissalicylic acid disodium salt;Olsalazine sodium, >=98%;Benzoic acid, 3,3-(1,2-diazenediyl)bis[6-hydroxy-, sodium salt (1:2);4,4′-Dihydroxyazobenzene-3,3′-dicarboxylic acid disodium salt;5,5′-Azobis(salicylic acid, sodium salt);Benzoicacid,3,3’-azobis[6-hydroxy-,disodiumsalt | | CAS: | 6054-98-4 | | MF: | C14H11N2NaO6 | | MW: | 326.24 | | EINECS: | 227-975-7 | | Product Categories: | Intermediates & Fine Chemicals;Pharmaceuticals;Aromatics;Pharmaceutical intermediate;API;AZODISAL SODIUM | | Mol File: | 6054-98-4.mol |  |
| | Olsalazine sodium Chemical Properties |
| | Olsalazine sodium Usage And Synthesis |
| Treatment of acute and chronic colitis | Olsalazine sodium is a drug developed by Pharmacia A B Co., Switzerland, to treat acute and chronic colitis. It was first listed in Denmark in 1989. It has been collected and recordedby the European Pharmacopoeia 7 edition and the national drug standard WS1(X-349) -2004Z. Orsalazine sodium is a precursor drug consisting of two azo bonds linked to 5amino salicylic acid. The bacteria in the colon break up the diazo bond and release 5amino salicylic acid. 5amino salicylic acid acts on colitis mucosa, inhibits the formation of prostaglandins, leukotrienes and other inflammatory factors, and reduces membrane permeability, and plays a role in the treatment of ulcerative colitis. This product weakens the absorption of nitrogen, sodium and water in the ileum and colonic mucosa, which is transformed into secretion in human body. Patients with ulcerative colitis take 1g daily, and the whole intestinal transit time is shortened by 40%. If the healthy subjects are given oral administration of 2g once, It has no effect on the complex frequency of migration of the small intestine. But it can cause diarrhea in 30% of the users and increase the diarrhea of the patients with intestinal inflammation. The bioavailability of the whole body is extremely low. The absorption rate of the oral dose is less than 5%.
After absorption, about 10% doses will be conjugated to orsalazine sulfate, t1/2 about 1H and conjugated half life is about 7 days.
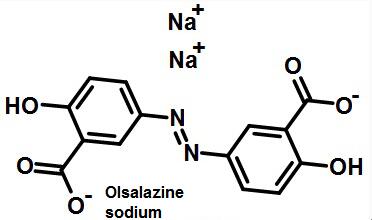
Figure 1 The structural formula of oralalazine sodium | | Adverse reaction | The most common side effect is diarrhoea, the incidence of which is 17%. Usually, the difference between diarrhea and intestinal inflammation is that the excreta has high water content without blood, and is related to the dosage. It usually occurs when the treatment begins or the dose increases. Reduction the dose of this product or combined use with Loperamide, diarrhea will be controlled. Other side effects include headache, nausea, abdominal pain, rash, dizziness and joint pain. Most of the patients who are allergic or intolerant to sulfasalazine are resistant to this product. The male sterility treated with sulfasalazine improved after using this product.
| | Chemical Properties | Yellow Crystalline Powder | | Uses | Dimer of Mesalamine. It is an anti-inflammatorydrug used in the treatment of inflammatory bowel disease and ulcerative colitis | | Uses | Dimer of Mesalazine (M258100), an anti-inflammatory drug used in the treatment of inflammatory bowel disease and ulcerative colitis. | | Uses | Mordant dye for wool. | | Preparation | 5-Amino-2-chlorobenzoic acid diazotization, coupled with 2-Hydroxybenzoic acid, and then in a certain pressure and contains trace copper powder or Cupric oxide of Sodium hydroxide solution common heating (130 ~ 150 ℃). | | Definition | ChEBI: An organic sodium salt that is the disodium salt of 3,3'-azobis(6-hydroxybenzoic acid) (olsalazine). Effective in the treatment of inflammatory bowel disease and ulcerative colitis. Mechanism of action unknown, but appears to be topical | | Brand name | Dipentum (UCB). | | Clinical Use | Induction and maintenance of remission in ulcerative
colitis | | Metabolism | Olsalazine is broken down by the colonic bacterial flora
into 2 molecules of 5-aminosalicylic acid (mesalazine).
The small amounts (1-2% of the dose or less) of intact
olsalazine that are absorbed are excreted mainly in urine.
Approximately 0.1% of an oral dose of olsalazine
is hepatically metabolised to olsalazine-O-sulfate
(olsalazine-S), which has a half-life of 7 days. | | Properties and Applications | deep green light yellow. Soluble in water for green light yellow, moderate soluble in Etanol for green yellow. In concentrated sulfuric acid for yellow orange, dilution after light yellow, In concentrated nitric acid to red. Dye aqueous solution to join concentrated hydrochloric acid color greatly shallow for yellow; Join concentrated Sodium hydroxide solution for golden orange.
|
Standard
|
Ironing Fastness
|
Light Fastness
|
Fulling
|
Persperation Fastness
|
Soaping
|
Water
|
|
Alkali
|
Acid
|
|
ISO
|
4-5
|
6
|
4-5
|
2-3
|
4-5
|
5
|
5
|
|
AATCC
|
5
|
6-7
|
4
|
|
5
|
5
|
5
|
|
| | Olsalazine sodium Preparation Products And Raw materials |
|



