|
| Product Name: | Pimecrolimus | | Synonyms: | ASM 981;Elidel;SDZ-ASM 981;Picrolimus;Pimecrolimus;15,19-Epoxy-3H-pyrido(2,1-C)(1,4)oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone, 3-((1E)-2-((1R,3R,4S)-4-chloro-3-methoxycyclohexyl)-1-methylethenyl)-8-ethyl-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26A-hexadecahydro-5,19-dihydroxy-14,16-dimethoxy-4,10,12,18-tetramethyl-, (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26as)-;15,19-Epoxy-3H-pyrido(2,1-C)(1,4)oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone, 3-(2-(4-chloro-3-methoxycyclohexyl)-1-methylethenyl)-8-ethyl-, 5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26A-hexadecahydro-5,19-dihydroxy-14,16-dimethoxy-4,10,12,18-tetramethyl-, (3S-(3R*(E(1S*,3S*,4R*)),4S*,5R*,8S*,9E,12R*,14R*,15S*,16R*,18S*,19S*,26ar*))-;Unii-7kyv510875 | | CAS: | 137071-32-0 | | MF: | C43H68ClNO11 | | MW: | 810.45 | | EINECS: | 603-999-7 | | Product Categories: | Inhibitors;Elidel, SDZ-ASM-981;Chiral Reagents;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 137071-32-0.mol |  |
| | Pimecrolimus Chemical Properties |
| Melting point | 135-136 °C | | Boiling point | 866.1±75.0 °C(Predicted) | | density | 1.19 | | storage temp. | Keep in dark place,Sealed in dry,2-8°C | | solubility | DMSO (Slightly, Sonicated), Chloroform (Slightly), Methanol (Slightly) | | pka | 9.97±0.70(Predicted) | | form | Solid | | color | White to Off-White |
| | Pimecrolimus Usage And Synthesis |
| Topical immunomodulators | Pimecrolimus is a topical immunomodulators and is a kind of semi-synthetic products of the Streptomyces-produced ascomycin. It has a greater lipophilicity than tacrolimus with a stronger affinity with the skin. It can effectively and safely control the signs and symptoms of facial seborrheic dermatitis and can quickly relieve the itching symptoms of patients with dermatitis and eczema as well as reduce the lesion area, clear atopic dermatitis (eczema) sign. Therefore, it is an effective treatment method for the treatment of atopic dermatitis (eczema) for both early remission purpose and long-term control purpose.
Seborrheic dermatitis is the body's immune inflammatory response to lipophilic Malassezia. Both the humoral and cellular immunity participate in the sickening process. Pimecrolimus can selectively inhibit the activation of T cell through inhibiting the activity of the calcium-dependent nerve transcription proteins which is indispensible for the activation of T cells and prevent the release of cytokines and pro-inflammatory mediator, and further exhibiting strong anti-inflammatory activity. These results suggest that the mechanism of pimecrolimus for treatment of seborrheic dermatitis comes from its anti-inflammatory effect and immune regulation. Currently there have been no reports regarding on its direct participation of antifungal activity. But in recent years, there have been reports finding that tacrolimus (a kind of immunomodulatory agents with similar chemical structure and mechanisms as pimecrolimus) have some anti-Malassezia activity.
Similar as the tacrolimus, pimecrolimus is a cellular selective inhibitor which is produced and released by the pro-inflammatory cytokine and can combine with macrophilin 12 (FKBP-12) to inhibit of calcium-dependent calcineurin as well as suppress the T-cell activation through blocking the early cytokines transcription of T cells. In particular, a level of nanogram is enough to suppress the synthesis of T cell IL-2 and IFN-γ (Th1 cell origin) factor and can also inhibit the synthesis of IL-4 and IL (Th2 cell-derived). In addition, in vitro experiments, pimecrolimus can also be applied to inhibit the antigen as well as IgE stimulated mast cells’ release of inflammatory cytokines and inflammatory mediators [4, 5]. Clinical data have shown that after topical application, the blood concentration is very low and is usually under the detected value (<0.5 ng/mL). It also has no drug accumulation phenomenon.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
| | Description | Pimecrolimus is an ascomycin
macrolactam derivative, developed as
a topical formulation (1% cream) for
the treatment of mild to moderate
atopic dermatitis for patients aged two
years and over in whom the use of
conventional therapies is inadvisable.
Pimecrolimus is an inflammatory
cytokine inhibitor that works by
selectively targeting T-cells in the skin.
It inhibits in vitro the production and
release of pro-inflammatory cytokines
after antigen-specific or non-specific stimulation in T cells and mast cells. Pimecrolimus
binds specifically to cytosolic receptor macrophilin-12 at nanomolar concentrations leading
to inhibition of the Ca2+/calmodulin-dependent phosphatase, calcineurin. Pimecrolimus is a
chlorine derivative of the known FK-520, from which it can be synthesized. In a pig model
of dinitrofluorobenzene-induced allergic contact dermatitis, pimecrolimus was shown to
inhibit erythema and induration, had equivalent efficacy compared to clobetasol-17-
propionate, but did not cause atrophogenic effects. In a mouse model of allergic contact
dermatitis, pimecrolimus given orally was as potent as tacrolimus and more effective than
cyclosporin. The agent also decreased the intensity of cutaneous manifestations in an
atopic dermatitis model involving hypomagnesemic hairless mice. Both in adults and in
pediatric patients with atopic dermatitis, treatment with pimecrolimus has demonstrated
greater efficiency than conventional treatment in reducing the incidence of disease flares,
as well as the use of second-line corticosteroids. Moreover, in a 26 week study, in pediatric
patients (2-17 years), from 65% of subjects showing improvement, 85% were cleared of the disease. Oral pimecrolimus administration resulted in an elimination half-life of about
30 to 40 h. It is not metabolized or degraded during skin permeation after topical
administration. However, following oral administration, it is metabolized via the liver
CYP3A4 pathway and excreted mainly in the feces. Pimecrolimus is well tolerated; no
systemic accumulation is seen and the most commonly reported side effect is reaction at
the site of application. Moreover, pimecrolimus did not cause skin atrophy in contrast to
corticosteroids. At this time, as a replacement therapy for topical corticosteroids, the only
competing agent for pimecrolimus is topical tacrolimus (ointment). Preliminary studies
demonstrated comparable efficacy and safety between these two agents. | | Chemical Properties | White Solid | | Originator | Novartis (USA) | | Uses | Pimecrolimus is a semi-synthetic, macrocyclic lactone derived from ascomycin by activation of the 32-hydroxy group with a triflate ester, and nucleophilic substitution with chloride under phase transfer conditions to provide the chloro analogue. Pimecrolimus has been targeted for treatment of inflammatory skin disorders. Like all tacrolimus analogues, pimecrolimus binds to receptor protein, FKBP12. The complex then binds to mTOR preventing it from interacting with target proteins. Pimecrolimus is extensively cited in the literature with over 2,000 citations. | | Uses | Macrolactam ascomycin derivative; inhibits production of pro-inflammatory cytokines by T cells and mast cells. Immunosuppresant Pimecrolimus caused a strong and dose-dependent inhibition of anti-IgE–induced release of histamine from mast cells and basophils (maximally 73% and 82%, respectively, at 500 nmol/L pimecrolimus) and of mast cell tryptase (maximally 75%) and a less pronou. | | Uses | Pimercrolimus (21) is the first non-steroid agent for the
treatment of mild to moderate atopic dermatitis lunched by
Novartis. It selectively blocks the production and release of
cytokines from T-cells. These cytokines cause inflammation,
redness and itching associated with eczema. Long-term
therapy with pimecrolimus (21) was more effective than
conventional treatment in reducing the incidence of disease
flares and the use of corticosteroids. This drug is also safe
and effective in pediatric patients and is approved for use in
children as young as two years. | | Indications | Pimecrolimus (SDZ ASM 981, Elidel) is another recently
approved macrolide immunosuppressant that
acts by inhibiting calcineurin and blocking the release
of proinflammatory cytokines from T lymphocytes. The
parent compound, ascomycin, was originally isolated
from Streptomyces hygroscopicus var ascomyceticus.
Like tacrolimus, pimecrolimus is approved for the topical
treatment of moderate to severe atopic dermatitis
that is refractory to other therapies.Transient local irritation
is a common side effect. | | Definition | ChEBI: Pimecrolimus is a lactam and a macrolide. | | Brand name | Elidel (Novartis);Elide1. | | Synthesis | The syntheses of
pimecrolimus (21) appeared in several patent applications. Starting material 178 was prepared by either
fermentation or modification of a previously described
synthetic method in the literature. Treatment of macrolide 178 with triisopropylsilyl trifluoromethanesulfonate
(TIPS-triflate) in the presence of lutidine in DCM
at 0??C afforded di-protected compound 179 in 94% yield.
Selective deprotection of the TIPS group at position 32
using p-TSA in MeOH at rt gave mono-protected macrolide
180 in 88% yield. Reaction of the hydroxyl group at
position 32 with o-nitrobenzenesulfonyl chloride (181) in
the presence of DMAP and DIPEA in DCM provided 182 in
78% yield with 20% recovered starting material 180.
Displacement of the sulfate with chloride using LiCl in
DMF furnished the chlorinated compound, which was
treated with aqueous HF to remove the TIPS group to
provide pimecrolimus (21). 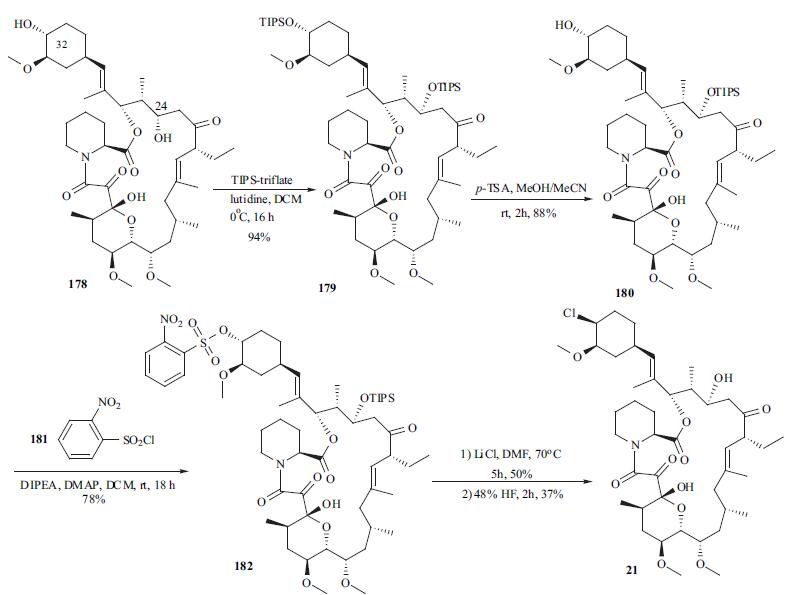
| | Veterinary Drugs and Treatments | A relatively new addition to the human topical armamentarium, pimecrolimus cream may be of benefit in veterinary patients in the
adjunctive treatment of atopic dermatitis, discoid lupus erythematosus, pemphigus erythematosus or foliaceous, pinnal vascular disease,
alopecia areata, vitiligo and for perianal fistulas (terminal phase or maintenance treatment after cyclosporine therapy). Unlike topical
corticosteroids, tacrolimus or pimecrolimus do not have atrophogenic or metabolic effects associated with long-term or large area
treatment.
Pimecrolimus acts similarly as cyclosporine and tacrolimus, namely inhibiting T-lymphocyte activation primarily by inhibiting the
phosphatase activity of calcineurin. It also inhibits the release of inflammatory cytokines and mediators from mast cells and basophils.
Pimecrolimus may not have identical mechanisms of action as tacrolimus, as it did not impair the primary immune response (as did
tacrolimus) in mice after a contact sensitizer was applied. Both drugs did impair the secondary response however. Any clinical significance
associated with this difference is not yet clear. | | references | [1]. stuetz a, grassberger m, meingassner jg. pimecrolimus (elidel, sdz asm 981)--preclinical pharmacologic profile and skin selectivity. semin cutan med surg, 2001, 20(4): 233-241.
[2]. zuberbier t, chong su, grunow k, et al. the ascomycin macrolactam pimecrolimus (elidel, sdz asm 981) is a potent inhibitor of mediator release from human dermal mast cells and peripheral blood basophils. j allergy clin immunol, 2001, 108(2): 275-280.
[3]. kalthoff fs, chung j, stuetz a. pimecrolimus inhibits up-regulation of ox40 and synthesis of inflammatory cytokines upon secondary t cell activation by allogeneic dendritic cells. clin exp immunol, 2002, 130(1): 85-92. |
| | Pimecrolimus Preparation Products And Raw materials |
| Raw materials | Methanesulfonic acid, trifluoro-, 4-[2-(8-ethyl-1,4,5,6,7,8,11,12,13,14,15,16,17,18,19,20,21,23,24,25,26,26a-docosahydro-5,19-dihydroxy-14,16-dimethoxy-4,10,12,18-tetramethyl-1,7,20,21-tetraoxo-15,19-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclotricosin-3-yl)-1-propenyl]-2-methoxycyclohexyl ester, [3S-[3R*... |
|



