|
| | SUGAMMADEX Basic information |
| Product Name: | SUGAMMADEX | | Synonyms: | Shu Glucose;SUGAMMADEX;Org 25969;Sugammadex [inn];Unii-361lpm2T56;SugaMMadex SodiuM;Sugammagex;Bridion | | CAS: | 343306-71-8 | | MF: | C72H112O48S8 | | MW: | 2002.15 | | EINECS: | 253-874-2 | | Product Categories: | | | Mol File: | 343306-71-8.mol |  |
| | SUGAMMADEX Chemical Properties |
| Melting point | >208°C (dec.) | | density | 1.559±0.06 g/cm3(Predicted) | | storage temp. | Inert atmosphere,Room Temperature | | solubility | Aqueous Acid (Slightly), Water (Slightly, Sonicated) | | form | Solid | | pka | 3.42±0.10(Predicted) | | color | White to Pale Beige | | Stability: | Hygroscopic |
| | SUGAMMADEX Usage And Synthesis |
| Description | Sugammadex is a selective relaxant binding agent indicated for reversal of neuromuscular blockade induced by rocuronium bromide and vecuronium bromide during surgery in adults. Rocuronium bromide and vecuronium bromide are neuromuscular blocking medications that cause temporary paralysis and are especially useful for general anesthesia, ventilation, or tracheal intubation that patients may require for surgery. Sugammadex provides a new treatment option to reverse the effects of those medications and possibly help patients recover sooner post-surgery. Sugammadex (brand name Bridion) is marketed by Merck Sharp and Dohme, and was approved by the United States FDA on December 15, 2015. | | Uses | Reversal agent for neuromuscular blocking drugs. | | Uses | 6A,6B,6C,6D,6E,6F,6G,6H-octakis-S-(2-carboxyethyl)-6A,6B,6C,6D,6E,6F,6G,6H-octathio-γ-Cyclodextrin, also known as Sugammadex, is used to reverse postoperative residual neuromuscular blockade. | | Definition | ChEBI: An octasaccharide derivative that is gamma-cyclodextrin in which all eight primary hydroxy groups are replaced by 2-(carboxyethyl)sulfanyl groups. Used (as the octasodium salt) for reversal of neuromuscular blockade induced by rocuronium and
vecuronium in adults undergoing surgery. | | Synthesis | Bromination
of |?-cyclodexdrin 127 with the Vilsmeier-Haack reagent
prepared by reaction of bromine with triphenylphospine
in DMF gave the per-6-bromo-|?-cyclodextrin 128 in 95-98%
yield. Nucleophilic displacement of the bromines of 128
with methyl 3-mercaptopropionate (129) and cesium carbon-ate at 50??C in DMF gave 6-perdeoxy-6-per(2-methoxycarbonylethyl)
thio-|?-cyclodextrin 130 as a white powder.
Saponification of the esters of 130 was accomplished by
reaction with aqueous sodium hydroxide solution to provide
sugammadex (XVII) as a glassy solid in 52% yield for the 2
steps. 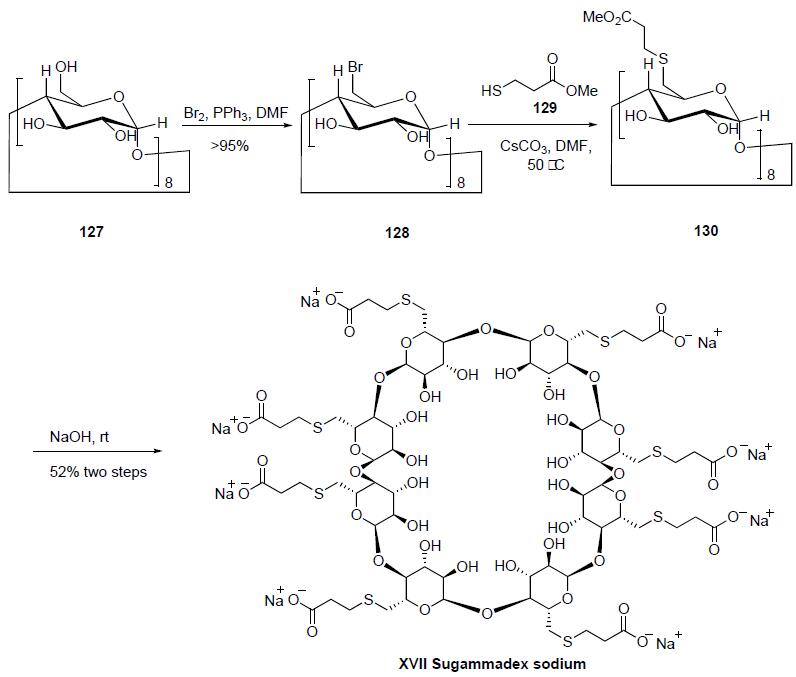
|
| | SUGAMMADEX Preparation Products And Raw materials |
|