| Chemical Properties | Yellow-brown, granular, free-flowing
polymer.Combustible. |
| Chemical Properties | Sodium polysulphides have the general formula Na2Sx where x can be 2-6.
All of these compounds have been prepared in the solid state, in which they
are well defined. |
| Uses | The sodium polysulphide solutions used for the preparation of polysulphide
elastomers generally have a rank of appro'ximately 2; for elastomers
with a high sulphur content which have especially good solvent resistance, a
rank of about 4 is used. In general, the rank of a polymer is close to that of the
sodium polysulphide solution from which it is formed. |
| Uses | Manufacture of sulfur dyes and colors, insec-
ticides, oil-resistant synthetic rubber (“Thiokol”
[Toray]), petroleum additives, electroplating.
|
| Preparation | Sodium polysulphide solutions are most commonly prepared by treating
sulphur with aqueous sodium hydroxide:
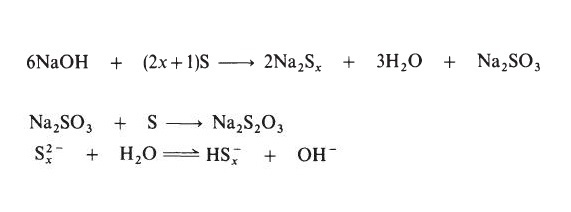
|
| Flammability and Explosibility | Nonflammable |