|
| | sacubitril Basic information |
| Product Name: | sacubitril | | Synonyms: | sacubitril;(S)-5-[(biphenyl-4-yl)Methyl]pyrrolidin-2-one;(S)-5-([1,1'-biphenyl]-4-ylMethyl)pyrrolidin-2-one;2-Pyrrolidinone, 5-([1,1'-biphenyl]-4-ylmethyl)-, (5S)-;sacubitril ISO 9001:2015 REACH | | CAS: | 1038924-61-6 | | MF: | C17H17NO | | MW: | 251.32 | | EINECS: | | | Product Categories: | | | Mol File: | 1038924-61-6.mol |  |
| | sacubitril Chemical Properties |
| Boiling point | 473.6±24.0 °C(Predicted) | | density | 1.117±0.06 g/cm3(Predicted) | | pka | 16.31±0.40(Predicted) |
| | sacubitril Usage And Synthesis |
| Description | Sacubitril is a neprilysin
inhibitor prodrug developed by Novartis that was approved
as part of an orally administered supramolecular sodium salt
complex with the angiotensin receptor blocker (ARB) valsartan
in the U.S. and EU in 2015. Sacubitril/valsartan (also known
as LCZ-696) is a first-in-class dual angiotensin receptor
blocker neprilysin inhibitor (ARNI) marketed for the treatment
of chronic heart failure with reduced ejection fraction
(HFrEF). It represents a novel mechanistic approach to
targeting HFrEF and is the first pharmacologic agent approved
for HFrEF since 2004. Sacubitril is metabolized by enzymatic
conversion of the ethyl ester to the active diacid (LBQ-657,
structure not disclosed), which inhibits neprilysin and prevents endogenous natriuretic peptide degradation. Neprilysin
inhibitors like sacubitril are not effective as monotherapy and
need to be combined with a reninangiotensin aldosterone
system (RAAS) inhibitor such as valsartan. Notably, dual
neprilysin and angiotensin-converting enzyme (ACE) inhibition,
as in omapatrilat, was found to be associated with an
increased risk of life-threatening angioedema due to increased
bradykinin levels. In phase III clinical trials, sacubitril/
valsartan displayed a superior safety profile to enalapril, with
a 20% decrease in heart failure hospitalizations or cardiovascular
death and a 16% reduction in the risk of death from any
cause. Sacubitril/valsartan is now recommended as the standard
of care for HFrEF as an alternative to ACEs and ARBs. | | Synthesis | Several routes to sacubitril, particularly to advanced
intermediates, have been published in the primary and patent
literature.23 They differ generally in their choice of chiral pool
starting material and their approach to introduction of the
second stereocenter. The industrial scale synthesis of
intermediate 47 has been reported. Accordingly, addition of the cuprate of biaryl
bromide 41 to (S)-epichlorohydrin 42 followed by subjection
to HCl provided chloropropanol 43 in 92% yield and 99% ee.
Next, a Mitsunobu reaction involving succinimide 44 followed
by treatment with refluxing HCl and NaOH generated the
corresponding aminoalcohol, which was isolated via crystallization
as the HCl salt prior to Boc protection to give N-Boc
aminoalcohol 45 in >99% ee. Alcohol 45 was then carried
through a four-step process to give acid 47 in 75% yield,
starting with oxidation of the alcohol to the corresponding
aldehyde with TEMPO/NaOCl. The organic phase was carried
forward directly into a Wittig reaction with ylide 46, generating
an |á,|?-unsaturated ester which was hydrolyzed to acid 47 with
LiOH in an ethanol/water mixture. Interestingly, a separate
patent disclosed the stereoselective hydrogenation of the
trisubstituted olefin 47, in which subjection of 47 to catalytic
[Ru(p-cymene)I2]2 and chiral phosphine ligand Mandyphos
SL-M004-1 (48) under 40 bar of hydrogen gas in warm ethanol
delivered 49 in 99:1 dr before recrystallization.
Subsequently, activation of the acid as the acid halide through
the use of thionyl chloride and ethanol not only reestablished
the ethyl ester but removed the Boc group, revealing a primary
amine which then reacted with succinic anhydride to ultimately
deliver sacubitril (V). The freebase form of sacubitril does not
readily crystallize; the isolation of a number of pharmaceutically acceptable salts of sacubitril via crystallization, most preferably
the calcium salt 50 or sodium salts, have been reported.
Preparation of the sacubitril/valsartan supramolecular complex
(trisodium salt, hemihydrate) has been described on a kilo-scale
from sacubitril calcium salt via neutralization to the freebase
and subsequent complexation with valsartan in iPrOAc/
acetone. Addition of NaOH and crystallization then provided
the desired trisodium salt hemihydrate.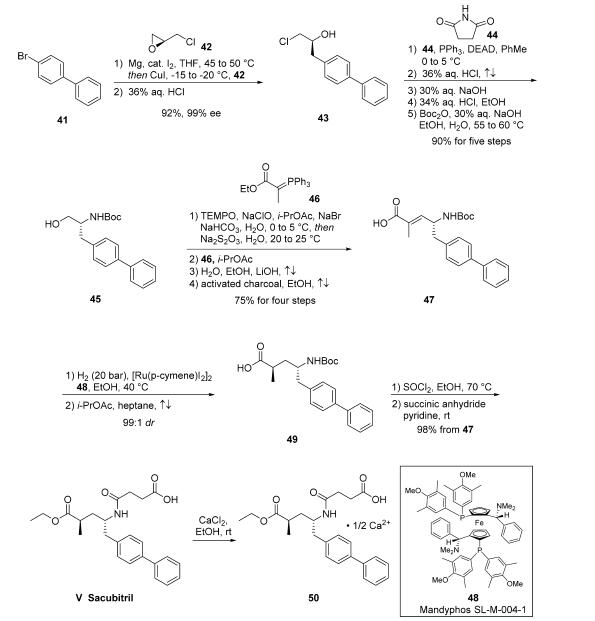
|
| | sacubitril Preparation Products And Raw materials |
|



