|
| | Enzalutamide Chemical Properties |
| Melting point | 198 - 200°C | | density | 1.49 | | storage temp. | -20°C | | solubility | Soluble in DMSO (>25 mg/ml) | | form | solid | | pka | 13.88±0.46(Predicted) | | color | White to off-white | | Stability: | Stable for 1 year as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| | Enzalutamide Usage And Synthesis |
| Pharmacological action | Enzalutamide (XTANDI) is an oral androgen receptor antagonist, which is approved by the current clinical research and the US FDA (Food and Drug Administration), for the development of post-chemotherapy metastatic castration tolerance of prostate cancer Treatment (i.e. patients with prostate cancer after chemotherapy, cancer or cancer cells are still growing in such patients), can extend the survival of patients. The incidence of prostate cancer in the United States is very high, with an annual increase of nearly 240,000 patients (the highest among all cancers), nearly 30,000 deaths every year (second only to lung cancer, breast cancer, colorectal cancer),but its incidence is low in China.
Enzalutamide is an androgen receptor inhibitor, the target of action is different from the approved cabbitaxel in 2010 and the approved abbitolone in 2011, and can competitively inhibit androgen and receptor binding, and can inhibition of androgen receptor nuclear transport and the interaction of the receptor and DNA. Vitro studies have shown that enzalutamide can inhibit prostate cancer cell proliferation and induce death, and enzalutamide reduces tumor volume in mouse prostate cancer xenograft model experiment. The main metabolite of enzalutamide is N-demethylol enamine, which shows similar inhibitory activity with enzalutamide in vitro. The recommended adult dose for the drug is daily 160mg, rapidly absorbed after medication, plasma concentrations to reach the highest level in 0.5~3h, the average terminal half-life is 5.8d, the main metabolic enzymes are CYP2C8 and CYP3A4. The drug should be avoided with strong CYP2C8 inhibitors (such as gemfibrozil), if it is needed for co-administration, should reduce the dose of enzalutamide to 80mg, 1 day. | | Treatment | In vitro, enzalutamide suppressed proliferation and induced apoptosis in human prostate cancer cell lines. The sensitivity of prostate cancer cells to T cell-mediated lysis via androgen receptor-dependent immunomodulation was enhanced by enzalutamide. Enzalutamide lacked androgen receptor agonist activity in CRPC cell models and induced tumour regression in CRPC xenograft models.
| | Indications | For the treatment of metastatic or recurrence of advanced male castration tolerance of prostate cancer. | | Clinical evaluation | Enzalutamide is an oral androgen receptor inhibitor that reduces the risk of radiation-related disease progression and death.
Although anti-androgen therapy has been the preferred treatment option for patients with metastatic prostate cancer for more than 70 years, researchers have found that male hormone receptors play an important role in the development of prostate cancer throughout the course of disease with the in-depth exploration of disease at the molecular level. FDA-approved new generation of androgen receptor antagonists such as abiraterone and enzolatamide have shown that it is benefit for patients with advanced prostate cancer after chemotherapy with docetaxel.
Astellas in Japan and Johnson in the United States treat prostate cancer both through the target, according to statistics, similar to the treatment results, only a few differences, such as: abitron to be used simultaneously with steroid drugs , and enzalutamide is not required; abitron need to limit the diet, and enzalutamide does not need. Drugs in different countries is not the same as different the crowd, so there are price differences, but the effect is the same.
This information was compiled by the Editor of the Chemicalbook (2015-09-20). | | Side effects | The main side effects of enxtran (XTANDI) are hypertension and fatigue. The most common adverse reactions (≥ 5%) are weakness/fatigue, back pain, diarrhea, joint pain, hot flashes, peripheral blood edema, musculoskeletal pain, headache, upper respiratory tract infection, muscle weakness, dizziness, insomnia, lower respiratory tract infection, spinal cord compression syndrome and cauda equina syndrome, hematuria, paresthesia, anxiety and hypertension. | | Biological activity | Enzalutamide (MDV3100) is an androgen receptor (AR) antagonist with an IC50 of 36 nM.
Enzalutamide is an androgen receptor (AR) antagonist with an IC50 of 36 nM in vitro studies. In competitive experiments with 16β-[18F] fluoro-5α-DHT (18-FDHT), enzalutamide was found to have higher affinity for bilirubin than Bicalutamide when administered to AR. While enzalutamide had no effect on LNCaP/AR (AR-overexpression) of prostate cells. In parental LNCaP cells, Enzalutamide inhibits the production of prostate specific antigen (PSA) and transmembrane serine protease 2 (TMPRSS2), and inhibits their binding to the synthetic and rogen R1881. Enzalutamide inhibits the translation activity of the mutant AR protein (W741C, Trp at position 741 mutanting to Cys). MDV310 also blocked the nuclear translocation and coordination receptor complexes that recruit coactivators. | | In vivo studies | Enzalutamide treatment of castrated male mice carrying LNCa/AR xenografts, mice treated with 10 mg MDV310 per kilogram of body weight, can induce significant tumor regression. | | Description | In August 2012, the US FDA approved enzalutamide for the treatment of
metastatic castration-resistant prostate cancer (mCRPC) in patients who have
previously been treated with docetaxel. Synthesis of enzalutamide was achieved by a triply convergent route that employed a
Strecker condensation, followed by isothiocyanate condensation and
hydrolysis to form the thiohydantoin moiety. In LNCaP/AR cells with
high expression of AR, enzalutamide demonstrated potent inhibition of
16b-[18F]-5α-dihydrotestosterone binding (IC50=21 nM compared with bicalutamide IC50=160 nM), and inhibited AR translocation to the nucleus more potently than bicalutamide.The primary metabolite is the result of CYP2C8-mediated N-demethylation; enzalutamide is primarily eliminated by hepatic metabolism. | | Chemical Properties | White Solid | | Originator | University of California (United States) | | Uses | MDV3100, known as Enzalutamide, is a second-generation androgen receptor (AR) signaling inhibitor. It is able to inhibit binding of androgens to the AR, AR nuclear translocation, and the association of the AR with DNA.
| | Definition | ChEBI: A benzamide obtained by formal condensation of the carboxy group of 4-{3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl}-2-fluorobenzoic acid with methylamine. Used for the treatment of of metastatic castration-resistant p
ostate cancer. | | Brand name | Xtandi | | Clinical Use | In August 2012, the FDA approved enzalutamide, marketed by Medivation and Astellas Pharma US
for the treatment of metastatic castration-resistant prostate cancer (CRPC), specifically for those patients
who had previously received docetaxel. Enzalutamide is an inhibitor of androgen receptors (AR)—
whose increased expression has been closely linked with castration-resistant prostate cancer (CRPC),thus, AR inhibitors have seen increased recent attention from the medicinal chemistry community.
Phase I/II trials were particularly promising for enzalutamide, as 43% of patients showed >50%
sustained suppression of a key serum biomarker. | | Synthesis | Of the several patents and papers describing
synthetic approaches, a 2011 patent represents the most likely scale production route to
enzalutamide, and this is described in the scheme.Commercially available carboxylic acid 70 was first converted to the corresponding acid chloride 71,
followed by amide formation with methylamine to furnish benzamide 72 in 90% yield over two steps.
Bromide 72 was then coupled with amine 73 using copper (I) catalysis to afford trisubstituted benzene
74 in 76% isolated yield. Esterification of 74 to 75 with iodomethane furnished one fragment for the
key ring-forming event. Isothiocyanate 76, available in one step from the corresponding aniline 77, was
then exposed to aminoester 75 in the presence of warm isopropyl acetate, resulting in construction of the
lynchpin thiohydantoin and delivering enzalutamide (XI) in an impressive 78% yield. This 5-step
process has successfully generated multi-gram quantities of the drug in 50.7% overall yield. 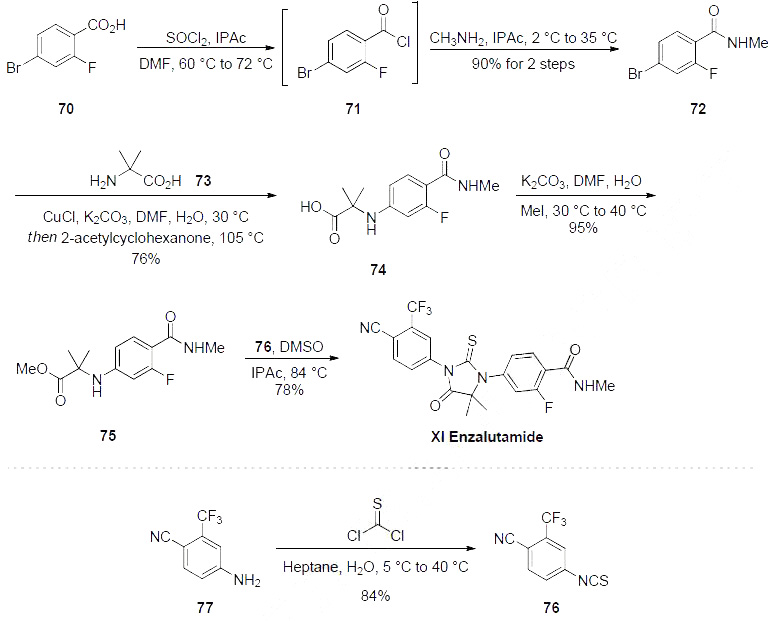
| | target | Androgen-receptor | | Drug interactions | Potentially hazardous interactions with other drugs
Anticoagulants: possibly reduces concentration effect
of coumarinsAnxiolytics: concentration of midazolam reduced.Cytotoxics: concentration of palbociclib possibly
reduced - avoid. Lipid-regulating drugs: concentration increased by
gemfibrozil - avoid or halve enzalutamide dose. | | Metabolism | Clearance of enzalutamide is mainly via hepatic
metabolism, producing an active metabolite that is equally
as active as enzalutamide and circulates at approximately
the same plasma concentration as enzalutamide. Under
conditions of clinical use, enzalutamide is a strong
inducer of CYP3A4, a moderate inducer of CYP2C9
and CYP2C19, and has no clinically relevant effect on
CYP2C8Excreted mainly as metabolites 71% in urine and 14% via
faeces. | | References | Tran et al. (2009), Development of a Second-Generation Antiandrogen for Treatment of Advanced Prostate Cancer; Science 324 787
Jung et al. (2010), Structure-Activity Relationship for Thiohydantoin Androgen Receptor Antagonist for Castration-Resistant Prostate Cancer (CRPC); J. Med. Chem. 53 2779 |
| | Enzalutamide Preparation Products And Raw materials |
|



