|
| | Pazufloxacin mesilate Basic information |
| | Pazufloxacin mesilate Chemical Properties |
| Melting point | >255°C (dec.) | | alpha | D20 -64.2° (c = 1 in 1.0N NaOH) | | storage temp. | -20°C Freezer | | solubility | Aqueous Base (Slightly), DMSO (Slightly) | | form | Solid | | color | Off-White to Pale Yellow | | CAS DataBase Reference | 163680-77-1(CAS DataBase Reference) |
| | Pazufloxacin mesilate Usage And Synthesis |
| Description | This fluoroquinolone was co-developed by Toyama and
Mitsubishi Pharm and was launched for the intravenous
therapy of respiratory, urinary, surgical, gynecological and
systemic infections. | | Description | Pazufloxacin is a broad-spectrum synthetic fluoroquinolone antibiotic. It is active against Gram-negative and Gram-positive bacteria, including clinical isolates of E. coli, K. pneumoniae, methicillin-susceptible and -resistant S. aureus, and methicillin-susceptible and -resistant S. epidermidis (MIC90s = 0.05, 0.1, 0.39, 12.5, 0.39, and 6.25 μg/ml, respectively). It inhibits E. coli, P. aeruginosa, and S. aureus DNA gyrase (IC50s = 0.88, 1.9, and 10.2 μg/ml, respectively) and S. aureus topoisomerase IV (IC50 = 24.2 μg/ml) in cell-free assays. In vivo, pazufloxacin is active against systemic E. coli, K. pneumoniae, and methicillin-resistant S. aureus infections in mice (ED50s = 0.15, 5.5, and 4.5 mg/kg, respectively). | | Chemical Properties | Crystalline Solid | | Uses | A fluorinated quinolone antibiotic. Antibacterial. | | Synthesis | Pazufloxacin Mesilate is elegantly synthesized from commercially available 2,3,4,5-tetrafluorobenzoic acid
(168) by an 11-step process with an overall yield 48% [68].
Starting material 168 was first treated with ethyl bromide
and then with t-butyl cyanoacetate in the presence of
potassium carbonate in DMSO in one flask to give acylated
cyanoacetate 169. Intermediate 169 thus obtained without
purification was refluxed in toluene with p-TSA to yield 4-
cyanomethylbenzoate 170 in 90% yield from 168.
Cyclopropanation at the benzylic position of 170 was
performed by |á,|á-dialkylation with two equiv. of 1,2-
dibromoethane under phase-transfer conditions to give
cyanocyclopropyl compound 171. Cyano compound 171
was subjected to hydration with alkaline H2O2 to afford
carboxamide 172 in 81% yield from 170. Subsequently,
carboxamide 172 was treated with NaOCl for Hofmann
rearrangement to give primary amine 173, which was
protected as its N-acetyl derivative 174 for the next reaction.
Treatment of 174 with imidazole in the presence of thionyl
chloride and TEA generated an imidazolide intermediate,
which was converted to |?-keto ester 175 by reacting with
potassium ethyl malonate and MgCl2. Enamine 176 was
obtained without purification by successive treatment of 175
with DMF-dimethylacetal and (S)-(+)-2-aminopropanol.
Crude 176 was heated in DMSO in the presence of
potassium carbonate to efficiently give tricycle product 177
in 80% yield from 174. Finally, the ethyl ester and acetamide in 177 were hydrolyzed under basic and acidic
conditions, respectively, to give the free amine. Pazufloxacin
mesilate (20) was obtained in 94% yield by treatment of its
corresponding free amine with methanesulfonic acid in
ethanol. 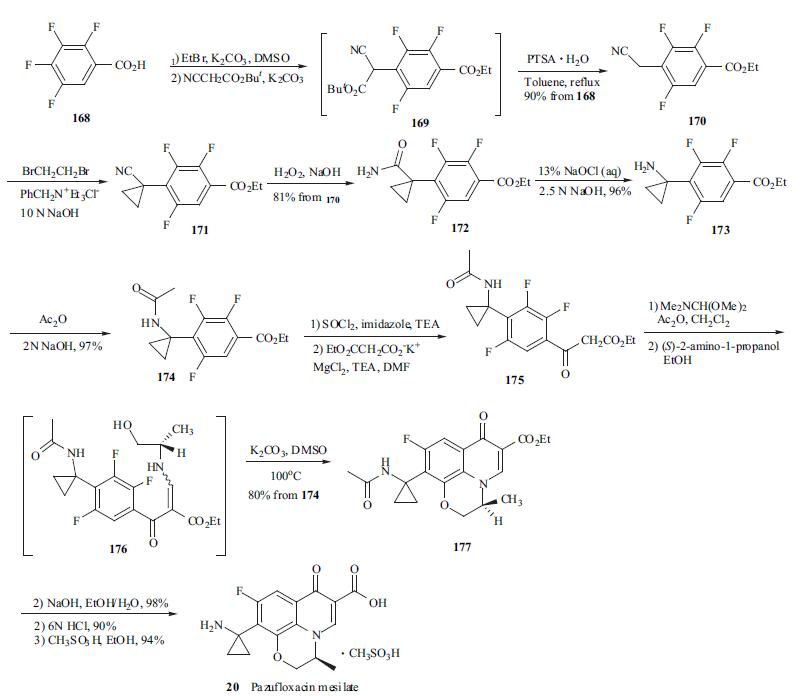
|
| | Pazufloxacin mesilate Preparation Products And Raw materials |
|