|
| | Phenacetin Chemical Properties |
| Melting point | 133-136 °C (lit.) | | Boiling point | 132 °C / 4mmHg | | density | 1.1248 (rough estimate) | | refractive index | 1.5710 | | Fp | 2℃ | | storage temp. | Sealed in dry,Room Temperature | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | powder | | pka | pKa 2.2(H2O) (Uncertain);3.5(aqueous acetone) (Uncertain) | | color | White | | Water Solubility | 0.076 g/100 mL | | Sensitive | Hygroscopic | | Merck | 14,7204 | | BRN | 1869238 | | Stability: | Stable. Incompatible with strong oxidizing agents, strong acids. | | InChIKey | CPJSUEIXXCENMM-UHFFFAOYSA-N | | LogP | 1.580 | | CAS DataBase Reference | 62-44-2(CAS DataBase Reference) | | IARC | 1 (Vol. 24, Sup 7, 100A) 2012 | | NIST Chemistry Reference | Acetamide, N-(4-ethoxyphenyl)-(62-44-2) | | EPA Substance Registry System | Phenacetin (62-44-2) |
| | Phenacetin Usage And Synthesis |
| Chemical Properties | Phenacetin occurs at room temperature as white, odorless monoclinic prisms. It is soluble in water (more so in hot than cold water), alcohol, glycerol, and acetone and is slightly soluble in benzene. It is unstable to oxidizing agents, iodine, and nitrating agents (IARC 1977). | | Uses | Phenacetin was used as an analgesic and fever-reducing drug in both human and veterinary medicine for many years. It was introduced into therapy in 1887 and was extensively used in analgesic mixtures until it was implicated in kidney disease (nephropathy) due to abuse of analgesics (Flower et al. 1985) and was withdrawn from the U.S. market in 1983 (Ronco and Flahault 1994, FDA 1998, 1999). Phenacetin also was previously used as a stabilizer for hydrogen peroxide in hair-bleaching preparations (IARC 1980, HSDB 2009). | | Indications and Usage | Phenacetin is mainly used as an antipyretic analgesic, with slow and lasting effects, treating headaches, neuralgia, joint pain, and fever, and weakly resisting rheumatism and inflammation. Because of toxic side effects and the rapid development of similar drugs, however, it is no longer used alone, only as a raw material in combination with other drugs. Commonly combined with aspirin and caffeine to form a less toxic compound aspirin used to treat the common cold. Can make chlorpheniramine cold tablets by adding a small amount of chlorpheniramine to the above compound, used to treat colds with headache, neuralgia, rheumatism, etc. Can be used as a material for organic synthesis or a pharmaceutical intermediate.
| | Mechanisms of Action | On its own, phenacetin has no antipyretic or analgesic effects. In vivo, acetaminophen and paracetamol are metabolized and decomposed to create the antipyretic and analgesic effects. Its decomposites with ammonia and phenyl either not only have no antipyretic and analgesic effects, but also are major factors in its side effects.
| | Side Effects | Long term use may cause renal papillary necrosis and interstitial nephritis, and even induce renal pelvic cancer and bladder cancer. Phenacetin also makes the hemoglobin to form methemoglobin, decreasing blood oxygen carrying capacity, causing cyanosis. In addition, Phenacetin can cause hemolysis and hemolytic anemia, and is toxic to the retina. Long term use may cause also lead to dependence. Countries including America, Britain, German, and Japan have banned Phenacetin, or required packaging to note that it is “not indicated for long-term usage or large doses.”
| | Carcinogenicity | Phenacetin is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Cancer Studies in Experimental Animals
Dietary administration of phenacetin caused benign and malignant tumors of the urinary tract in mice and rats of both sexes and of the nasal cavity (adenocarcinoma, squamous-cell carcinoma, and transitional-cell carcinoma) in rats of both sexes (Isaka et al. 1979, IARC 1980).
Cancer Studies in Humans
There is limited evidence for the carcinogenicity of phenacetin in humans. There are numerous case reports of kidney cancer (transitionalcell carcinoma of the renal pelvis) among patients who had consumed large amounts of analgesic mixtures containing phenacetin; however, it is not possible to specify which component(s) of the mixture is carcinogenic (IARC 1977, 1980).
https://ntp.niehs.nih.gov/ntp/roc/content/profiles/phenacetinandanalgesicmixtures.pdf
| | Synthesis of Phenacetin | The preparation of phenacetin is a straightforward, two-step “one-pot” organic synthesis.
In a 10-mL Erlenmeyer flask place 0.20 g (1.46 mmol) of p-phenetidine (MW 137) and 3.50 mL of water.
Add 4 drops only (about 1.0 mmol) of concentrated (12 M) hydrochloric acid, which should dissolve the amine completely. Do not be concerned if some undissolved material remains. Add a spatula-tip of activated carbon (decolorizing charcoal) to the solution, swirl the solution on a hot plate for a few minutes, and remove the charcoal by pipette filtration into a clean 10-mL flask.
Note: If a very slight color persists in the pphenetidine hydrochloride solution, do not repeat the decolorizing procedure but continue with the remainder of the experiment. If the solution is fairly dark, however, a second decolorizing charcoal treatment may be necessary.
Prepare a weakly basic solution by dissolving 0.24 g (2.92 mmol) of sodium acetate (MW 82) in 0.80 mL of water in a 10-mL flask. Set this solution aside for later use.
Warm the p-phenetidine hydrochloride solution on a hot plate. Add 0.20 mL (0.22 g, 2.20 mmol) of acetic anhydride (MW 102) while swirling the solution. Add the sodium acetate solution all at once, and swirl the solution vigorously to insure mixing. Allow the solution to stand at room temperature for 5 minutes. If no crystals form, add 1 or 2 drops of acetic anhydride.
Cool the reaction mixture by immersing the flask in an ice-water bath, and swirl the mixture vigorously until the crude phenacetin crystallizes. Collect the crystals by suction filtration. Wash the crystals with a portion of cold water.
Purify the crystals by recrystallization from water. Collect the crystals by suction filtration and air dry the crystals.
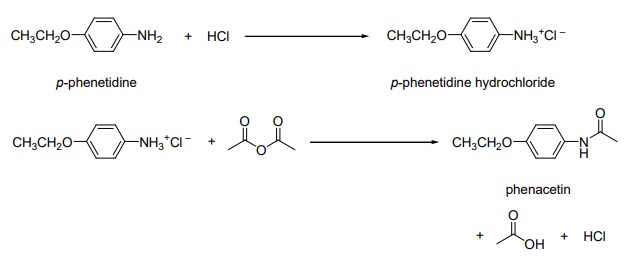
https://www.stolaf.edu/people/hansonr/chem253/expt4_2008_phenacetin.pdf
| | Description | Phenacetin, a painkiller, was the world’s first synthetic pharmaceutical
drug. It was one of the first painkillers that was not
derived from opium while at the same time being absent of antiinflammatory
qualities. Phenacetinwas developed in 1878 by an
American chemist, Harmon Northrop Morse. It was introduced
into the pharmaceutical market in 1887. However, it was withdrawn
in 1983 in the United States due to unacceptable levels of
interstitial nephritis in patients and potential risks of tumorigenicity.
Like in the United States, most Western countries did not
ban phenacetin from marketing until 1983. Phenacetin is
a component of APC (aspirin-phenacetin-caffeine). | | Chemical Properties | Acetophenetidin is a fine, white, crystalline powder or solid. Odorless with a slightly bitter taste | | Originator | Phenacetin ,Environmental Health | | Uses | Analgesic, antipyretic. Component of APC tablets, analgesic mixture also containing aspirin and caffeine.
Phenacetin is reasonably anticipated to be a human carcinogen; analgesic mixtures containing Phenacetin are listed as known human carcinogens. | | Uses | glycosylation inhibitor | | Uses | Phenacetin was used as an analgesic and fever-reducing drug in
both human and veterinary medicine for many years until it
was implicated in kidney disease (nephropathy) due to abuse
of analgesics and was withdrawn from the market. Phenacetin
also was previously used as a stabilizer for hydrogen peroxide
in hair-bleaching preparations. | | Definition | ChEBI: Phenacetin is a member of the class of acetamides that is acetamide in which one of the hydrogens attached to the nitrogen is substituted by a 4-ethoxyphenyl group. It has a role as a non-narcotic analgesic, a peripheral nervous system drug and a cyclooxygenase 3 inhibitor. It is a member of acetamides and an aromatic ether. It is functionally related to a N-phenylacetamide, a 4-ethoxyaniline and a paracetamol. | | Manufacturing Process | A mixture of 10 g of 4-ethoxyaniline and 8.6 g of acetic anhydride in 28 g of dry benzene was refluxed for 4 hours. To the reaction mixture was added a small amount of Na2S2O4. After cooling the phenacetin was crystallized; yield 12.5 g (96%), M.P. 136°C. | | Brand name | [Names previously used: Acetophenetidin;
Acetphenetidin.];292-comprimes 369, pulvules 3p bugesic;Acetylosal;Acifein;Acromas;Acropac;Algocratine;Alumidyne;Amypron;Amypylo-n;Angifebrine;Anodin;Antiflu des;Apadine;Apidin;Apracur;Arcin;Asceine;Ascophen;Ascthimindon;Asfeen;Ban-o-pain;Bexophene;Bromo quinina;Butal compound;Butorinal;Calmante muri;Capacetyl;Capramin;Caps dr knapp;Capsula dr. knapp;Ceachin;Cefinal;Cequinyl fort;Chloracet;Citramol;Codopyrin;Codral;Conta-schmerz;Coricidin f;Cotradol;Darvocomp-n;Darvon compuesto 65;Darvon n compuesto;Dentocaps;Dolafort;Dolomo;Doloxene comp forte, capsules;Dolviron;Doregrippin;Doscafis;Doviron;Drinacet;Estrifen;Femcaps;Fenascor;Fenbutal;Flexalgit;Florital;Fonal;Fridol;Friocellin;Funapann;Gripanidan;Harbureta;Hemagene taylor;Icn 65;Influenza tabs;Isollyl;Isomidon;Katagrip;Lekasin;Linarol;Manasul;Mardon;Migesic;Mironal;Monacet;Myolate;Neopyrine;Nevral vit b1 b6;Novacetol;Novosephalgin;Olfano;Omniadol;Papnin;Para-grip;Parametten;Pargesic compound;Pasadex;Pedigel;Phenacetine powder;Phenorial;Polypyrine;Poxy;Procomp-65;Prodigestan;Prodolor;Protension;Quadrochin;Rectoral;Refagan;Repro;Respritin;Rhinazol;Rinurel;Rinutan;Robaxisan-pm;Ron-drive;Rumicine;S antineuralgic;S fc;Sacadol;Sadaspir;Sedalmerck;Sk 65 compound caps.;Soma compound;Soma compuesto;Sonalgin;Spacin;Spasmindon;Spasmo-compralgyl;Synalogos-dc;T h;Tetrex-apc;Tetrracydin;Tiiomapirina;Tomapiena;Triplex;Uga-no;Vandar-65;Vasogesic;Vicks action 500;Zactirin compound-100. | | Therapeutic Function | Analgesic | | World Health Organization (WHO) | Phenacetin, an aniline derivative, was introduced into medicine
as an antipyretic over a century ago. It subsequently gained recognition as an
analgesic and was available in many proprietary analgesic preparations. However,
in the 1940s its habitual use was first implicated as the cause of
methaemoglobinaemia and chronic haemolysis. Since 1950 there have been many
reports published indicating that abusive use is associated with cumulative renal
damage. Evidence also exists to suggest that it may have a carcinogenic potential.
The drug has been withdrawn in many countries but may remain available in others.
(Reference: (WHODI) WHO Drug Information, 1, 5, 1980) | | Synthesis Reference(s) | Synthesis, p. 168, 1995 DOI: 10.1055/s-1995-3868 | | General Description | Phenacetin is an odorless fine white crystalline solid with a lightly bitter taste. Used as an analgesic medicine. | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | Phenacetin react with oxidizing agents, iodine and nitrating agents. | | Fire Hazard | Flash point data for Phenacetin are not available but Phenacetin is probably combustible. | | Biochem/physiol Actions | Substrate of CYP1A2 and CYP2D6. | | Safety Profile | Confirmed carcinogen producing tumors of the lildney and bladder. A human poison by an unspecified route. Poison by intravenous and possibly other routes. Moderately toxic by several routes. Human systemic effects by ingestion: cyanosis, liver damage, and methemoglobinemiacarboxyhemo-globinemia. Experimental teratogenic data. Other experimental reproductive effects. Mutation data reported. Chronic effects consist of weight loss, insomnia, shortness of breath, weakness, and often aplastic anemia. When heated to decomposition it emits toxic fumes of NOx,. | | Potential Exposure | Phenacetin is used as an analgesic and antipyretic drug. It is used alone or in combination with aspirin and caffeine for mild to moderate muscle pain relief. Phenacetin has also been used as a stabilizer for hydrogen peroxide in hair bleaching preparations. A laboratory reagent. In veterinary medicine; it is used as an analgesic and antipyretic.
| | Carcinogenicity | Phenacetin is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. | | Environmental Fate | Phenacetin occurs at room temperature as white, odorless
monoclinic prisms. It is soluble in water, alcohol, glycerol, and
acetone and is slightly soluble in benzene. It is unstable to oxidizing agents, iodine, and nitrating agents. Phenacetin has
a melting point of 134–135 °C; log Kow of 1.58; water solubility
of 30 mg l-1 at 25 °C; and vapor pressure of 0.00316mmHg at
25 °C.
Phenacetin’s former use and production as an analgesic may
have allowed release into the environment through various
waste streams. Phenacetin exists both as vapor and as particulate
if released to air. The vapor phase is expected to be readily
degraded by reaction with photochemically produced hydroxyl
radicals with a half-life reaction of 22 h. The particular phase,
however, is removed by wet and dry deposition reactions.
Phenacetin can enter the environment through leaching into
groundwater when released into the soil with moderate
mobility. When released into the water, it does not adsorb to
suspended solids and sediment, but is expected to be inert to
reaction with naturally occurring oxidants found in water with
a half-life of more than 30 days. Phenacetin has an estimated
bioconcentration factor of less than 100, and is not expected to
significantly bioaccumulate. Volatilization is insignificant. | | Shipping | UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. | | Purification Methods | Crystallise it from H2O or EtOH, and its solubility in H2O is 0.08% (at ~10o) and 1.2% (at ~100o), and in EtOH it is 6.7% (at ~10o) and 36% (at ~100o). Alternatively it can be purified by solution in cold dilute alkali and re-precipitating by addition of acid to neutralisation point. Dry it in air. [Beilstein 13 H 461, 13 IV 1092.] | | Toxicity evaluation | It is unclear how phenacetin induces nephropathy. Studies
proposed that phenacetin’s metabolite, acetaminophen (paracetamol),
leads to lipid peroxidation that damages kidney cells
through cyclooxygenases reaction that catalyzes the conversion
of paracetamol into N-acetyl-p-benzoquinoneimine (NAPQI).
NAPQI, in turn, depletes glutathione via nonenzymatic
conjugation to glutathione, a naturally occurring antioxidant.With the depletion of glutathione, kidney cells are more
susceptible to oxidative damage. | | Incompatibilities | Oxidizing agents, iodine and nitrating agents. | | Waste Disposal | It is inappropriate and possibly dangerous to the environment to dispose of expired or waste pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible, return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged, and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Permanganate oxidation, microwave plasma treatment, alkaline hydrolysis or incineration. |
| | Phenacetin Preparation Products And Raw materials |
| Raw materials | Acetic acid-->Acetic anhydride-->Acetaminophen-->Iodoethane-->Phenetidine-->phenylacetic anhydride-->Sodium dithionite | | Preparation Products | 4-ETHOXY-N-METHYLACETANILIDE-->3,4-Dimethoxyphenol-->Phenacetin-d3-->Pyrrolo[2,3-b]indole, 5-ethoxy-1,2,3,3a,8,8a-hexahydro-1,3a,8-trimethyl-, (3aR,8aS)-rel--->Benzothiazole, 6-ethoxy-2-methyl- (7CI,8CI,9CI) |
|



