|
| | 3,5-dihydroxy-3-methyl-Pentanoic acid Basic information |
| | 3,5-dihydroxy-3-methyl-Pentanoic acid Chemical Properties |
| storage temp. | 2-8°C | | solubility | Soluble in DMSO | | form | neat | | Melting point | 151-152 °C(Solv: benzene (71-43-2)) | | pKa | 4.33±0.10(Predicted) | | Boiling point | 364.1±32.0 °C(Predicted) | | density | 1.263±0.06 g/cm3(Predicted) | | Concentration | 0.1 mCi/ml | | Solvent | Ethanol:0.15N NH4OH (1:1) | | Specific Activity | 10-20 Ci/mmol | | EPA Substance Registry System | Pentanoic acid, 3,5-dihydroxy-3-methyl- (150-97-0) |
| | 3,5-dihydroxy-3-methyl-Pentanoic acid Usage And Synthesis |
| Uses | Precursor in the biosynthesis of cholesterol. Occurs in equilibrium with the d-lactone | | Definition | Anorganic acid, it is an intermediate in the biosyn-thesis of squalene, cholesterol, and coenzyme q inplants and animals. | | Biosynthesis | Mevalonic acid is the primary precursor of an the terpenoids and steroids biosynthesised by plants. It is derived from acetyl CoA through the intermediate formation of acetoacetyl CoA and 3-hydroxy-3-methylglutaryl CoA (HMG CoA), these reactions being catalysed by acetyl CoA acetyltransferase and HMG CoA synthase, respectively. Reduction of HMG CoA, catalysed by HMG CoA reductase, gives mevalonic acid.
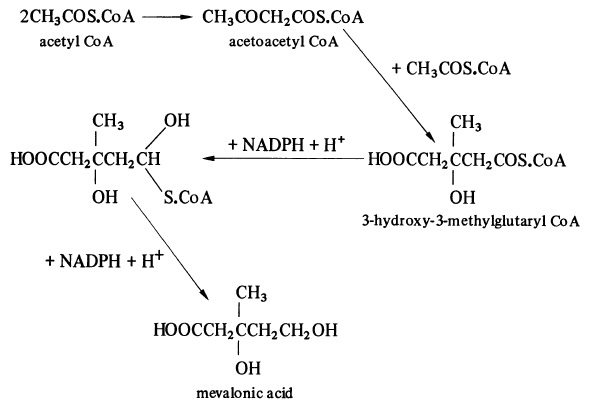
Before the pathway can continue, mevalonic acid must be catalytically phosphorylated by ATP and mevalonic acid kinase. Mevalonic acid kinase activity has been detected in many plants and has been found to be inhibited by such products of the acetate-mevalonate pathway as geranyl, farnesyl, geranylgeranyl and phytyl pyrophosphates. Thus, phosphorylation of mevalonic acid is a primary point at which control of terpenoid and steroid biosynthesis operates. Phosphorylation leads first to the mono- and then to the pyrophosphate. The second phosphorylation is catalysed by phosphomevalonate kinase. | | Biochem/physiol Actions | Metabolite of the mevalonate pathway, which plays a key role in the biosynthesis of sterols, dolichol, heme and ubiquinone. Of interest for research in the disease areas oncology, autoimmune diseases, artherosclerosis and Alzheimer disease, as well as for inherited deficiencies of mevalonate kinase. |
| | 3,5-dihydroxy-3-methyl-Pentanoic acid Preparation Products And Raw materials |
|