|
| | 4-TRIMETHYLSILYL-3-BUTYN-2-OL Basic information |
| | 4-TRIMETHYLSILYL-3-BUTYN-2-OL Chemical Properties |
| Boiling point | 76 °C | | density | 0.847 g/mL at 25 °C | | refractive index | n 20/D 1.446 | | Fp | 143 °F | | storage temp. | 2-8°C | | solubility | highly soluble in all standard organic solvents
(hexanes, toluene, CH2Cl2, EtOAc, alcohols, ethers). Partially
soluble in water. | | pka | 13.78±0.20(Predicted) | | form | clear liquid | | color | Colorless to Light orange to Yellow | | Specific Gravity | 0.846 | | Hydrolytic Sensitivity | 4: no reaction with water under neutral conditions | | BRN | 1923632 | | CAS DataBase Reference | 6999-19-5(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26 | | RIDADR | 1993 | | WGK Germany | 3 | | TSCA | No | | HazardClass | 3 | | PackingGroup | III | | HS Code | 29319090 |
| | 4-TRIMETHYLSILYL-3-BUTYN-2-OL Usage And Synthesis |
| Chemical Properties | Colorless liquid | | Physical properties | bp 83–85°C (13 mmHg). | | Uses | More recently, Mulzer has reported use of the corresponding
allenylsilane derived from 4-TMS-3-butyn-2-ol for use in the synthesis
of the C13–C18 fragment of branimycin (eq 2).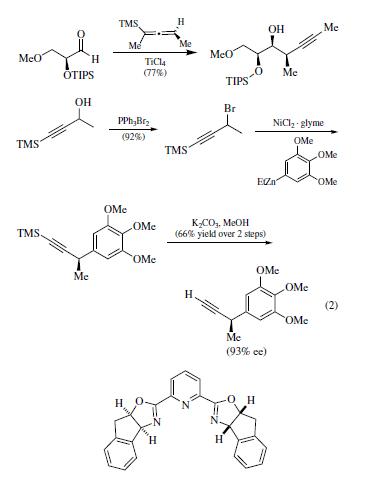 | | Preparation | racemic 4-trimethylsilyl-3-butyn-2-ol can
be prepared by deprotonation with strong bases (BuLi, LDA,
Grignards reagents) of trimethylsilylacetylene followed
by addition to acetaldehyde.Deprotonation of 3-butyn-2-
ol followed by quenching with excess trimethylsilyl chloride
followed by concomitant hydrolysis of the trimethylsilyl ether
is generally the most straightforward route.Enzymatic reduction
of 4-TMS-3-butyn-2-one has also been used to prepare the
reagent using alcohol dehydrogenase.
Preparation of nonracemic 4-TMS-3-butyn-2-ol has been
accomplished by asymmetric addition of dimethylzinc to
acetaldehyde promoted by TADDOL or addition of a
trimethylsilylvinylsulfoxide to acetaldehyde followed by thermal
elimination of the sulfoxide.Asymmetric reduction of 4-
TMS-3-butyn-2-one using stoichiometric reducing reagents,
catalytic transfer hydrodrogenation,and enzymatic reduction
with isolated protein or whole cells afford the 4-TMS-3-butyn-
2-ol with varying degrees of enantioenrichment.Enzymatic resolution by esterification of the racemic alcohol is the method
of choice for the large-scale preparation. |
| | 4-TRIMETHYLSILYL-3-BUTYN-2-OL Preparation Products And Raw materials |
|



