| | Oxygen Chemical Properties |
| Melting point | −218 °C(lit.) | | Boiling point | −183 °C(lit.) | | density | 1.429(0℃) | | vapor density | 1.11 (vs air) | | vapor pressure | >760 mmHg at 20 °C | | storage temp. | -20°C | | solubility | At 20 °C and at a pressure of 101 kPa, 1 volume dissolves in about 32 volumes of water. | | form | colorless gas | | color | Colorless gas, liquid, or hexagonal crystals | | Odor | Odorless gas | | Water Solubility | one vol gas dissolves in 32 volumes H2O (20°C), in 7 volumes alcohol (20°C); soluble other organic liq, usually higher solubility than in H2O [MER06] | | Merck | 13,7033 | | Stability: | Stable. Vigorously supports combustion. Incompatible with phosphorus, organic materials, many powdered metals. | | CAS DataBase Reference | 7782-44-7(CAS DataBase Reference) | | NIST Chemistry Reference | Oxygen(7782-44-7) | | EPA Substance Registry System | Oxygen (7782-44-7) |
| | Oxygen Usage And Synthesis |
| Description | Oxygen is a very prevalent and important element and is
necessary for sustaining life on this planet. This element is the
third most abundant in mass behind helium and hydrogen in
the universe. The diatomic form (O2) is the most common pure
form. With a boiling point at -183 °C, O2 exists as a colorless
and odorless gas at standard temperature and pressure. In the
process of cellular respiration, the highly reactive O2 is used as
the oxidant in breaking down food molecules to produce
energy. In turn, photosynthetic organisms generate O2 by using
energy from the sun and water. Other allotropes of pure oxygen
exist, including the trioxygen (O3) form known as ozone, as
well as other, less common allotropes of oxygen such as O4 and
O8. These oxygen allotropes are formed under high pressure
and low temperatures and are solid. | | Chemical Properties | Oxygen, O2, is a colorless, tasteless, gaseous element essential to almost all forms of life. It promotes respiration and combustion. Oxygen comprises 20% of the earth's atmosphere and is the most abundant element in seawater and in the earth's crust. It is slightly soluble in water and alcohol, but combines readily with most other elements to form oxides. The electrolysis of water produces both oxygen and hydrogen.
| | Physical properties | There are three allotropes (different forms) of oxygen: (1) atomic oxygen (O), sometimesreferred to as nascent or “newborn” oxygen; (2) diatomic oxygen (O2), or molecular oxygen(gas); and (3) ozone (O3), also a gas.
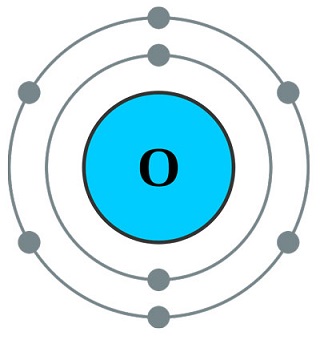
The atmospheric oxygen that we breathe is a very reactive nonmetal and is colorless, odorless,and tasteless, but it is essential to all living organisms. It readily forms compounds withmost other elements. With six electrons in its outer valence shell, it easily gains two moreelectrons to form a negative (–2) ion; or as covalent, it can share electrons with other elementsto complete its outer shell.
Almost all the oxygen in the atmosphere (?21%) is the allotropic form of molecular oxygen(O2). This essential gas we breathe is the result of photosynthesis, which is how green plants(with chlorophyll) use the energy of the sun to convert carbon dioxide (CO2) and water tostarches and sugars with molecular oxygen as the by-product.
Liquid oxygen has a slightly bluish cast to it. As it boils, pure oxygen gas is released. Themelting point for oxygen is –218.79°C, its boiling point is –182.95°C, and its density is0.001429 g/cm3. | | Isotopes | There are a total of 15 isotopes of oxygen, three of which are stable. The stableones are O-16, which accounts for 99.762% of all the oxygen on Earth; O-17, whichcontributes only 0.038% of the Earth’s oxygen; and O-18, which makes up just 0.200%of Earth’s oxygen. | | Occurrence | Oxygen is the third most abundant element in the universe, making up nearly half themass of the Earth’s crust and nine-tenths of the total mass of water. Even the mass of ourbodies consists of two-thirds oxygen. Oxygen is also the most abundant element in the Earth’satmosphere at 20.947% by volume.
Oxygen is produced commercially by liquefying air under reduced temperatures andincreased pressure. Then oxygen (and other gases) can be collected as the temperature rises inthe liquid air, allowing the various gases to boil off at their specific boiling points. This processis known as fractional distillation. Liquid air can be transported in vacuum vessels in theliquid form as long as there is a small vent to allow the escape of some of the gas that boilsaway as temperatures rise above the boiling point.
Fractional distillation is based on the principle that each element has its own temperatureat which it changes from a liquid to a gas. Thus, any gas can be separated from other liquefiedcomponents of air and then collected. The same process is used in the petroleum industry toseparate various fractions from the crude oil.
There are several methods of producing oxygen gas in the laboratory.
Oxygen can be produced by electrolysis of water using a salt as an electrolyte that produceshydrogen at the opposite electrode. When potassium chlorate (KClO3) is heated in a testtube with a small amount of manganese dioxide (MnO2) as a catalyst, the chemical reactionthat releases the oxygen from potassium chlorate will be accelerated. Use of potassium nitrate(KNO3) will also produce small amounts of oxygen.
A recent, and more productive, method is to pass air through fine molecular-size sievesof material that will absorb the nitrogen gas of air, which then allows the oxygen gas to passthrough the sieve to be collected. | | Characteristics | Oxygen is, without a doubt, the most essential element on Earth. It is required to supportall plant and animal life, and it forms more compounds with other elements than any otherelement.
Oxygen is soluble in both water and alcohol. Contrary to what many people believe, oxygenis NOT combustible (it will not burn), but rather it actively supports the combustion ofmany other substances. After all, if oxygen burned, every time a fire was lit, all the O2 in theatmosphere would be consumed!
Burning is a form of oxidation wherein oxygen chemically combines with a substance rapidlyenough to produce adequate heat to cause fire and light, or to maintain a fire once started.The oxidation of iron is called rusting. Rusting in an example of “slow oxidation,” which isthe reaction of O2 with Fe to form Fe2O3 or Fe3O4. This chemical reaction is so slow that theheat it produces is dissipated; thus, there is no fire.
Recently a new allotrope of oxygen was discovered. When O2 is subjected to great pressure,it is converted intoO4, which is a deep red solid that is a much more powerful oxidizer thanthe other forms of oxygen. | | History | Oxygen, as a gaseous element,
forms 21% of the atmosphere by volume from which
it can be obtained by liquefaction and fractional distillation.
The atmosphere of Mars contains about 0.15% oxygen. The
element and its compounds make up 49.2%, by weight, of
the Earth’s crust. About two thirds of the human body and
nine tenths of water is oxygen. In the laboratory it can be
prepared by the electrolysis of water or by heating potassium
chlorate with manganese dioxide as a catalyst. The gas is
colorless, odorless, and tasteless. The liquid and solid forms
are a pale blue color and are strongly paramagnetic. Ozone
(O3), a highly active compound, is formed by the action of an electrical discharge or ultraviolet light on oxygen. Ozone’s
presence in the atmosphere (amounting to the equivalent of
a layer 3 mm thick at ordinary pressures and temperatures)
is of vital importance in preventing harmful ultraviolet rays
of the sun from reaching the Earth’s surface. There has been
recent concern that pollutants in the atmosphere may have a
detrimental effect on this ozone layer. Ozone is toxic and exposure
should not exceed 0.2 mg/m3 (8-hour time-weighted
average — 40-hour work week). Undiluted ozone has a bluish
color. Liquid ozone is bluish black, and solid ozone is violet-
black. Oxygen is very reactive and capable of combining
with most elements. It is a component of hundreds of thousands
of organic compounds. It is essential for respiration of
all plants and animals and for practically all combustion. In
hospitals it is frequently used to aid respiration of patients.
Its atomic weight was used as a standard of comparison for
each of the other elements until 1961 when the International
Union of Pure and Applied Chemistry adopted carbon 12
as the new basis. Oxygen has thirteen recognized isotopes.
Natural oxygen is a mixture of three isotopes. Oxygen 18 occurs
naturally, is stable, and is available commercially. Water
(H2O with 1.5% 18O) is also available. Commercial oxygen
consumption in the U.S. is estimated to be 20 million short
tons per year and the demand is expected to increase substantially
in the next few years. Oxygen enrichment of steel
blast furnaces accounts for the greatest use of the gas. Large
quantities are also used in making synthesis gas for ammonia
and methanol, ethylene oxide, and for oxy-acetylene welding.
Air separation plants produce about 99% of the gas,
electrolysis plants about 1%. The gas costs 5¢/ft3 ($1.75/cu.
meter) in small quantities. | | Uses | In oxyhydrogen or oxyacetylene flame for welding metals and for lighting (calcium light, etc); submarine work by divers, propellant for rockets. In the production of synthesis gas which can be used in the Fischer-Tropsch process for liquid fuels. | | Uses | Oxygen has many uses due to its high electronegativity with the ability to oxidize manyother substances. Only fluorine has higher electronegativity and is thus a stronger oxidizer.Besides the essential use to support life, oxygen has many other uses.
It is used in the smelting process to free metals from their ores. It is particularly importantin the oxygen-converter process in the production of steel from iron ore.
Oxygen is used in making several important synthetic gases and in the production ofammonia, methyl alcohol, and so on.
It is the oxidizer for liquid rocket fuels, and as a gas, oxygen is used in a mixture withhelium to support the breathing of astronauts and divers and to aid patients who have difficultybreathing. It is use to treat (oxidize) sewage and industrial organic wastes.
Oxygen has many uses because of its ability to accept electrons from other elements to formionic bonds or to share electrons with other elements to form covalent bonds. | | Uses | The most obvious use of oxygen is to support life. The process
of aerobic respiration uses O2 in an oxidation reaction that
produces H2O and energy required for metabolic reactions.
Uses of commercially produced O2 gas are mainly industrial
and medical. O2 is a highly reactive element that reacts readily
with most other chemicals. The process of combustion uses
oxygen as the oxidizing element; for instance, the combustion
of hydrocarbons in cars requires O2 in a chemical reaction to
produce H2O, CO2, and energy as byproducts. Similarly,
rocket fuel containing H2 gas uses liquid O2 in a combustion
reaction to produce water vapor and heat. O2 is used in a wide
variety of commercial industries. Most commercially produced
O2 is used in the production of steel from iron ore. O2 is used
in the production of other metals as well, such as zinc, copper,
and lead. O2 is used in the gasification of coal to promote
more complete combustion in incinerators; in the production
of various chemicals such as ethylene oxide, propylene oxide,
and nitric acid; as a fuel component in blow torches for the
welding and cutting of metals. i.e., oxyacetylene torch; and in
the papermaking industry to bleach pulp. O2 is used in
wastewater treatment facilities and to reduce hydrogen sulfide
in sewers. O2 has many therapeutic uses in medicine. Highpurity
O2 gas is used in life support in surgeries, in intensive
care units to assist breathing, for premature babies, and with
inhalation therapies with chronic conditions. Hyperbaric
oxygen chambers provide oxygen at high partial pressures for numerous conditions, including instances of CO poisoning or
in treatment for decompression sickness, or ‘the bends,’ in
divers. | | Preparation | Most commercial oxygen at present is obtained from air by cryogenic separation processes. Although design of oxygen manufacturing plants and process conditions may vary depending on production capacity, purity desired, and cost, basic steps are similar.
Air first is filtered to remove dust particles. Water and carbon dioxide and most trace impurities are removed by silica gel (or other effective adsorbent) at a temperature slightly above 0°C. Acetylene and other hydrocarbons also can be removed by such adsorption processes. Alternatively, clean air is compressed and cooled to freeze out water and carbon dioxide, which can be trapped and removed in reversing exchangers. Compression and cooling of air is a critical step in its liquefaction. When cooled compressed air is allowed to expand it cools further (Joule-Thomson effect), converting the gaseous air to liquid air at about -196°C. Liquefied air is subjected to fractional distillation. More volatile argon and nitrogen distill out on warming, leaving behind oxygen with trace quantities of hydrogen, helium, and other inert gases.
Oxygen may be produced by electrolysis of water. In such electrolytic procedure, small amounts of H2SO4 or NaOH may be added to water. Electrolysis methods, however, are not used as much commercially as are air liquefaction processes which cost less. However, in making hydrogen from water by electrolysis, oxygen is obtained as a by-product.
In the laboratory oxygen may be prepared by several chemical methods that involve thermal decomposition of solid oxides or oxo salts. The most convenient method of preparing oxygen is to heat potassium chlorate in the presence of manganese dioxide catalyst:
Early preparation of oxygen involved thermal dissociation of metal oxides, notably mercury(II) oxide, which was used independently by both Priestley and Scheele. Also, oxides of lead, silver, and barium or potassium nitrate and permanganate were used by these and later investigators to prepare oxygen. Some reactions that yield oxygen by thermal decomposition of metal oxides and metal oxo salts, are highlighted below:
2HgO (s) → 2Hg (l) + O2 (g)
2BaO2 (s) → 2BaO (s) + O2 (g)
2PbO2 (s) → 2PbO (s) + O2 (g)
2KNO3 (s) → 2KNO2 (s) + O2 (g)
2Ag2O (s) → 4Ag (s) + O2 (g)
2KMnO4 (s) → K2MnO4 (s) + MnO2 (s) + O2 (g)
2K2S2O8 (s) → 2K2SO4 (s) + 2SO2 (g) + O2 (g)
Barium peroxide was used in commercial production of oxygen in the past. Heating barium oxide in air at 500°C forms barium peroxide, which decomposes at 800°C to yield oxygen:
Oxygen can be prepared chemically at ordinary temperatures. Several reactions in solution are known that may produce small quantities of oxygen at room temperatures. One such convenient method of producing oxygen is to slowly add water to sodium peroxide. The reaction is exothermic; therefore, the addition of water must be done cautiously.
2Na2O2 (s) + 2H2O (l) → 4NaOH (aq) + O2 (g)
Oxygen also is liberated when an acidified solution of potassium permangnate, acidified with sulfuric acid, is treated with a solution of hydrogen peroxide:
2MnO4ˉ (aq) + 5H2O2 (aq) + 6H+ (aq) → 2Mn2+ (aq) + 8H2O (l) + 5O2 (g). | | Production Methods | Oxygen is the most prevalent element in the Earth’s crust,
making up 49.2% by weight. It accounts for 20.95% by
volume of the Earth’s atmosphere and approximately 65% by
weight of the human body. | | Definition | Dioxygen: the normal form of molecularoxygen, O2, used to distinguishit from oxygen atoms or fromozone (O3). | | Definition | oxygen: Symbol O. A colourlessodourless gaseous element belongingto group 16 (formerly VIB) of the periodictable; a.n. 8; r.a.m. 15.9994; d.1.429 g dm–3; m.p. –218.4°C; b.p.–183°C. It is the most abundant elementin the earth’s crust (49.2% byweight) and is present in the atmosphere(28% by volume). Atmosphericoxygen is of vital importance for allorganisms that carry out aerobic respiration.For industrial purposes it isobtained by fractional distillation of liquid air. It is used in metallurgicalprocesses, in high-temperatureflames (e.g. for welding), and inbreathing apparatus. The commonform is diatomic (dioxygen, O2);there is also a reactive allotropeozone (O3). Chemically, oxygen reactswith most other elements formingoxides. The element wasdiscovered by Joseph Priestley in1774. | | Definition | The most abundant elementon earth, making up about 47% of the earth’s mass,and essential for respiration. | | General Description | Oxygen is a colorless, odorless and tasteless gas. Oxygen will support life. Oxygen is noncombustible, but will actively support the burning of combustible materials. Some materials that will not burn in air will burn in Oxygen. Materials that burn in air will burn more vigorously in Oxygen. As a non-liquid gas Oxygen is shipped at pressures of 2000 psig or above. Pure Oxygen is nonflammable. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. Oxygen is used in the production of synthesis gas from coal, for resuscitation and as an inhalant. | | Reactivity Profile | Propellant; ignites upon contact with alcohols, alkali metals, amines, ammonia, beryllium alkyls, boranes, dicyanogen, hydrazines, hydrocarbons, hydrogen, nitroalkanes, powdered metals, silanes, or thiols [Bretherick 1979. p.174]. Heat of water will vigorously vaporize liquid Oxygen, pressures may build to dangerous levels if this occurs in a closed container. Liquid Oxygen gives a detonable mixture when combined with powdered aluminum [NFPA 491M. 1991]. | | Hazard | Although oxygen itself is not flammable or explosive, as is sometimes believed, its mainhazard is that, in high concentrations, oxygen can cause other materials to burn much morerapidly.
Oxygen is toxic and deadly to breathe when in a pure state at elevated pressures. In addition,such pure oxygen promotes rapid combustion and can produce devastating fires, such asthe fire that killed the Apollo 1 crew on a test launch pad in 1967. It spread rapidly because thepure oxygen was at normal pressure rather than the one-third pressure used during flight.
Oxygen used for therapeutic purposes in adults can cause convulsions if the concentrationis too high. At one time, high levels of oxygen were given to premature infants to assist theirbreathing. It was soon discovered that a high concentration of O2 caused blindness in some ofthe infants. This practice has been abandoned, or the oxygen levels have since been reduced,and this is no longer a medical problem.
Oxygen involved in metabolic processes are prone to form “free radicals,” which arethought to cause damage to cells and possibly be associated with cancer and aging. | | Health Hazard | Inhalation of 100% Oxygen can cause nausea, dizziness, irritation of lungs, pulmonary edema, pneumonia, and collapse. Liquid may cause frostbite of eyes and skin. | | Health Hazard | Oxygen is nontoxic under the usual conditions of laboratory use. Breathing pure
oxygen at one atmosphere may produce cough and chest pains within 8 to 24 h, and
concentrations of 60% may produce these symptoms in several days. Liquid oxygen
can cause severe "burns" and tissue damage on contact with the skin due to extreme
cold. | | Fire Hazard | Oxygen itself is nonflammable, but at concentrations greater than 25% supports and
vigorously accelerates the combustion of flammable materials. Some materials
(including metals) that are noncombustible in air will burn in the presence of oxygen. | | Fire Hazard | Behavior in Fire: Increases intensity of any fire. Mixtures of liquid Oxygen and any fuel are highly explosive. | | Flammability and Explosibility | Oxygen itself is nonflammable, but at concentrations greater than 25% supports and
vigorously accelerates the combustion of flammable materials. Some materials
(including metals) that are noncombustible in air will burn in the presence of oxygen. | | Agricultural Uses | Oxygen (O) is an odorless, colorless, gaseous element
that belongs to group 16 (formerly group VI) of the
Periodic Table. It is the most abundant element
in the earth's crust (49.2% by weight), is present in the
atmosphere (20% by volume) and is a constituent of
water. It exists in three isotopes 16, 17 and 18. Oxygen is
essential for respiration of most living organisms and for
combustion. It is used in metallurgical processes, in high
temperature flames (welding) and in medical treatment.
The common form of oxygen is di-atomic oxygen
(O2) There is also another form - reactive allotrope
ozone (O3)C.h emically, oxygen reacts with most other
elements forming oxides. For industrial use, it is
obtained by fractional distillation of liquid air. This has
been replaced by a process which utilizes ambient
temperature separation by means of a pressure cycle in
which molecular sieves of synthetic zeolite preferentially
absorb nitrogen from air, giving 95 % oxygen and 5 %
argon.
The most popular industrial use of oxygen is in
oxygen enrichment of steel blast furnaces. Large
quantities of oxygen are used in the synthesis of nitric
acid from ammonia, methanol and ethylene oxide, as also
in oxy-acetylene welding. | | Safety Profile | Human systemic effects by inhalation: cough and other pulmonary changes. Human teratogenic effects by inhalation: developmental abnormalities of the fetal cardovascular system. Mutation data reported. Not toxic as gas. In liquid form it can cause severe "burns" and tissue damage on contact with the slun due to extreme cold. An oxidant. Though itself nonflammable,it is essential to combustion. Even a slight increase in the oxygen content of the air above the normal 21% greatly increases the oxidation or burning rate (and the hazard) of many materials. Exclusion of O2 from the neighborhood of a fire is one of the principal methods of extinguishment. Avoid smoking, flames, electric sparks. Liquid O2 can explode on contact with readdy oxidizable materials, especially at high temperatures. Under the proper condltions of temperature, pressure, and reagent concentration it can react violently with acetaldehyde, acetylene, acetone, secondary alcohols (e.g., 2-propanol, 2-butanol) aluminum, Al(BH4)3, AH3, aluminumtitanium alloys, alkali metals @hum, cesium, potassium, rubidlum, sodlum, potassium), ammonia, ammonia + platinum, asphalt, ccl4, chlorinated hydrocarbons, cyanogen, barium, benzene, 1,4-benzenediol + 1-propanol, benzoic acid, Be(BH4)2, biological materials + ether, BAszBr3, B2H10, diH6, boron tribromide, boron trichloride, bromine + chlorotrifluoroethylene, butane + Ni(CO)4, carbon disulfide, carbon disulfide + mercury + anthracene, carbon monoxide, CsH, calcium, calcium hosphide, copper + hydrogen sulfide, Cl0H14, cyclohexane-l,2-done biskhenylhydrazone), cycl0℃tatetraene, dborane, diboron tetrafluoride, dimethoxymethane, dimethylketene, dimethyl sulfide, diphenyl ethylene, disilane, ethers (e.g., diethyl ether, diisopropyl ether, tetrahydro furan, dtoxane, ethyl ether), fibrous fabrics, fluorine + hydrogen, fuels, germanium, glycerol, halocarbons (e.g., l,l,l-trichloroethane, trichloroethylene, chlorotrifluoroethylene, bromotrifluoroethylene), hydrazine, hydrocarbons (e.g., 1,l-dphenylethylene, gasoline, cyclohexane, ethylene, cumene, pxylene, but-3-yne), hydrocarbons + promoters (e.g., methyl nitrate, nitromethane, ethyl nitrate, tetrafluorohydrazine), hydrogen, hydrogen sulfide, lithiated dalkylnitrosoamines, magnesium, metals, metal hydrides (e.g., sodtum hydride, uranium hydride, lithium h ydride, potassium hydride , rubidium hydride, cesium hydride, magnesium hydride), methane, methoxycycl0℃tatetraene, 4-methoxytoluene7 Ni(CO)4 + butane, nonmetal hydrides (e.g., diborane, tetraborane(lO), phosphine, pentaborane(1 l), pentaborane(9) , decaborane(l4), aluminum tetrahydroborate), oil films, organic matter, (OF2 + H20), phosphorus, phosphorus tribromide, phosphorus trifluoride, phosphorus(IⅡ) oxide, polymers [e.g., foam rubber, neoprene, polytetrafluoroethylene (teflon)], polytetrafluoroethylene + stainless steel, polyurethane, polyvinyl chloride, propylene oxide, K2O2, rhenium, trirhenium nonachloride, rubber + ozone, rubberized fabric, selenium, NaH, sodium hydroxide + tetramethyldsiloxane, strontium, tetracarbonylnickel, tetracarbonylnickel + mercury, tetrafluoroethylene, tetrafluorohydrazine, tetrasilane, titanium and alloys, trisilane, CH2Cl2, oil, paraformaldehyde, wood, charcoal. Compressed O2 is shipped in steel cylinders under hgh pressure. If these containers are broken due to shock or exposed to high temperature, an explosion and fire may result.
| | Potential Exposure | Compressed oxygen is used in various
oxidation processes, for feedstock; and enrichment purposes;
as a medicinal gas; a chemical intermediate; in oxyacetylene
welding; in metallurgy. Liquid oxygen is used as
a rocket fuel. Oxygen is naturally present at a concentration
of 21% in breathing air. | | Carcinogenicity | Exposure to ionizing radiation is
recognized as a cause of cancer, and the production of
free radicals in the exposed cells is a part of the process.
If hyperoxia causes free radical formation, it may contribute
to the occurrence of cancer. Although a direct relation of
hyperoxic injury and cancer has not been proven, numerous
instances of association in experimental animals exist. Reactive
oxidative intermediates have been shown to cause chromosome
breaks and damage to DNA that can initiate
carcinogenesis.
Experimental work done with mouse skin tumors (as
models of human tumors) has been both revealing and
confusing. A substance may act as a cancer initiator or as
a promoter, or sometimes both, depending on intensity and
duration of exposure, and the presence of other carcinogenic
materials. The same substance can also inhibit cancer
growth. Hyperoxia has clearly been involved in modifying
the course of tumor development, but the effects have
differed under varying circumstances. | | Environmental Fate | Atmospheric air contains 20.8% O2. Despite consumption of
O2 through respiration and oxidative processes, this concentration
remains constant, most likely due to the depleted O2
being replaced by plant-generated O2 in the photosynthetic
process. O2 does not bioaccumulate in organisms as pure
oxygen. | | Shipping | UN1072 Oxygen, compressed & UN1073
Oxygen, refrigerated liquid (cryogenic liquid), Hazard
Class: 2.2; Labels: 2.2-Nonflammable compressed gas; 5.1-
Oxidizer. Cylinders must be transported in a secure upright
position, in a well-ventilated truck. Protect cylinder and
labels from physical damage. The owner of the compressed
gas cylinder is the only entity allowed by federal law
(49CFR) to transport and refill them. It is a violation of
transportation regulations to refill compressed gas cylinders
without the express written permission of the owner. | | Purification Methods | Purify it by passing the gas over finely divided platinum at 673oK and Cu(II) oxide (see under nitrogen) at 973o, then condensed in a liquid N2-cooled trap. HIGHLY EXPLOSIVE in contact with organic matter. | | Toxicity evaluation | The lack of sufficient O2 delivery to tissues of the body and thus
insufficient supply of energy to support cellular processes is
known as hypoxia. The partial pressure of O2 that exists in air is
160mmHg, and hypoxic effects will start to occur at partial
pressures of 120 mmHg and below. Occupational Safety and
Health Administration standards require 19.5% oxygen. The
brain, with a high metabolic rate and no O2 reserve, is the most
susceptible to hypoxia. Complex physiological changes occur
in response to hypoxia, including an increase in heart and
respiratory rate and artery constriction, all to try to ensure
adequate tissue oxygenation. Depending on the rate and extent
of hypoxia onset, different symptoms will be prevalent with
loss of consciousness and cessation of respiration and heart
activity in extreme states of hypoxia below about 10% O2.
Hypoxia due to carbon monoxide poisoning is due to the
higher relative affinity of hemoglobin in red blood cells to CO
over O2, resulting in insufficient O2 delivery to metabolizing
tissues, with deleterious effects on the heart and the central
nervous system (CNS) being most problematic. The partial
reduction of molecular oxygen in biological systems produces
cytotoxic intermediates, including the superoxide, hydrogen
peroxide, and hydroxyl radical. The superoxide radical especially
plays a significant role in a number of pathophysiologic
states associated with oxygen toxicity. Reactive oxygen species
(ROS) cause lipid peroxidation in the cell membranes, leading
to cellular damage and death. The ROS generated can also lead
to the production of other free radicals like nitric oxide, peroxynitrite,
and trioxidane, which can cause damage to DNA
and other molecules. Oxygen radical scavengers such as
superoxide dismutase and catalase protect the body against
oxygen-free radicals produced in day-to-day metabolic
reactions. | | Incompatibilities | A strong oxidizer. Reacts violently with
nearly every element, combustibles, organics, and reducing
materials. | | Waste Disposal | Return refillable compressed
gas cylinders to supplier. Vent to atmosphere. |
| | Oxygen Preparation Products And Raw materials |
|



