|
| | Desoxycorticosterone Basic information |
| | Desoxycorticosterone Chemical Properties |
| Melting point | 138-144 °C | | alpha | 184 º (c=1, C2H5OH) | | Boiling point | 407.89°C (rough estimate) | | density | 1.0998 (rough estimate) | | refractive index | 1.5192 (estimate) | | Fp | 9℃ | | storage temp. | -20°C | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Solid | | pka | 12.98±0.10(Predicted) | | color | White to Pale Yellow | | Water Solubility | slightly soluble | | Merck | 13,2917 | | BRN | 2062123 | | LogP | 2.880 | | CAS DataBase Reference | 64-85-7(CAS DataBase Reference) | | NIST Chemistry Reference | Deoxycorticosterone(64-85-7) | | EPA Substance Registry System | Deoxycorticosterone (64-85-7) |
| | Desoxycorticosterone Usage And Synthesis |
| Chemical Properties | white to creamy-white crystalline powder | | Uses | A mineralocorticoid that occurs in adrenal cortex. It acts as a precursor to Aldosterone (A514700). | | Uses | Antiinflammatory;Corticoide | | Definition | ChEBI: A mineralocorticoid that is progesterone substituted at position 21 by a hydroxy group. | | General Description | 11-Deoxycorticosterone is a steroid hormone produced in the adrenal glands that acts as a precursor for the hormone aldosterone. Levels of 11-deoxycorticosterone are measured by LC-MS/MS to aid in diagnosing disorders of steroid synthesis, such as 11-hydroxylase deficiency and glucocorticoid responsive hyperaldosteronism. This Certified Spiking Solution? is suitable for use as starting material inlinearity standards, calibrators, and controls for numerous LC-MS/MS applications in endocrinology, clinical chemistry, and neonatal screening. | | Hazard | Toxic. | | Mechanism of action | Desoxycorticosterone causes an increase in reabsorption of sodium ions and excretion
of potassium ions from the renal tubules, which leads to increased tissue hydrophilicity.
This facilitates an elevated volume of plasma and increased arterial pressure. Muscle tonicity
and work capability are increased. It is used for an insufficiency of function of the adrenal
cortex, myasthenia, asthenia, adynamia, and overall muscle weakness. | | Synthesis | Desoxycorticosterone, 21-hydroxypregn-4-en-3,20-dione acetate
(27.2.6), is synthesized in a number of ways, the easiest of which being iodination of
progesterone at C21 in the methyl group, and subsequent reaction of the resulting
iodo-derivative 27.2.5 with potassium acetate, which leads to formation of the desired desoxycorticosterone
in the form of the acetate (27.2.6) . 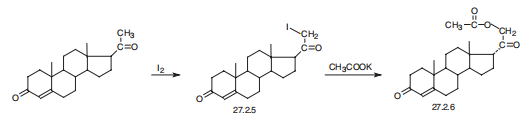
| | Purification Methods | Crystallise 11-deoxycorticosterone from diethyl ether. [Schindler et al. Helv Chim Acta 24 360 1941, Steiger & Reichstein Helv Chim Acta 20 1164 1937.] |
| | Desoxycorticosterone Preparation Products And Raw materials |
|



