|
| | Tiotropium bromide Basic information |
| Product Name: | Tiotropium bromide | | Synonyms: | Anhydrous Tiotropium Bromide;3-Oxa-9-azoniatricyclo[3.3.1.02,4]nonane, 7-[(2-hydroxy-2,2-di-2-thienylacetyl)oxy]-9,9-dimethyl-, bromide (1:1), (1α,2β,4β,5α,7β)-;(1α,2β,4β,5α,7β-7-[(Hydroxydi-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.0^<2,4>^]nonane bromide;Ba-679;Ba-679 BR;otropiuM broMide;(1R,2S,4R,5S,7R)-7-{[2-hydroxy-2,2-bis(thiophen-2-yl)acetyl]oxy}-9,9-diMethyl-3-oxa-9-azatricyclo[3.3.1.0^{2,4}]nonan-9-iuM broMide;TiotropiuM BroMide(Spiriva, BA 679BR) | | CAS: | 136310-93-5 | | MF: | C19H22BrNO4S2 | | MW: | 472.42 | | EINECS: | 680-665-7 | | Product Categories: | stock for supply, stable production, we can also offer commerical quantity;Tiotropium;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;Sulfur & Selenium Compounds;API;136310-93-5 | | Mol File: | 136310-93-5.mol |  |
| | Tiotropium bromide Chemical Properties |
| Melting point | 218-2200C | | storage temp. | Inert atmosphere,2-8°C | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | color | White to Off-White | | λmax | 240nm(lit.) | | Merck | 14,9454 | | CAS DataBase Reference | 136310-93-5(CAS DataBase Reference) |
| | Tiotropium bromide Usage And Synthesis |
| Description | Tiotropium bromide is a long-acting inhaled muscarinic antagonist, developed for the
once-daily treatment of chronic obstructive pulmonary disease. Tiotropium bromide can be
prepared in three steps. The Grignard condensation of 2-thienyl magnesium bromide with
oxalic acid dimethyl ester, followed by a transesterlfication with scopine provided the ester
which was quaternized with methyl bromide. Tiotropium bromide binds to human
recombinant muscarinic receptors M1-, M2- and M3-subtypes with high and similar affinity,
comparable to those obtained with ipratropium. Tiotropium bromide is characterized by its
novel property of kinetic selectivity : while ipratropium rapidly dissociated from each of the
receptor subtypes, tiotropium dissociated rapidly from M2 receptors (t1/2=3.6 h) but slowly
from MI (t1/2=14.6 h) and M3 (t1/2=34.7 h) receptors. Inhibition of cholinergic bronchospasm
by tiotropium bromide was demonstrated in anesthetized guinea pigs, rabbits and dogs. In
healthy volunteers, inhalation of tiotropium bromide resulted in an absolute bioavailability
of 19.5%, a t,,, value of 5 min. and the terminal half-life value of 5-6 days. There was no
evidence of drug accumulation after repeated administration. The extent of
biotransfonation was small with a urinary excretion of 74% of unchanged substance after
iv. administration. Long term studies in patients with stable COPD have demonstrated that
tiotropium bromide gave an effective bronchodilation that was maintained over 24h,
significantly improved lung function as measured by FEVI (+ll-12%) and showed
progressive reduction in dyspnea. It also reduced exacerbations of COPD patients and
improved quality of life. Tiotropium bromide produced greater and more sustained
bronchodilation than ipratropium bromide. Tiotropium has been shown to cause superior
bronchodilatation and symptomatic improvements when compared to twice daily
salmeterol in COPD. Tiotropium bromide was well tolerated and caused few adverse
effects. The most common side effect reported was the mechanism-related effect of dry
mouth. | | Description | Tiotropium is an antagonist that binds to M1, M2, and M3 muscarinic acetylcholine receptors (Kds = 0.43, 0.54, and 0.69 nM, respectively, for human receptors). It decreases acetylcholine-induced contraction of isolated guinea pig trachea in a concentration-dependent manner. In vivo, tiotropium (1 g/L inhaled aerosol) confers complete protection against acetylcholine-induced bronchospasms in anesthetized dogs. Formulations containing tiotropium have been used in the treatment of chronic obstructive pulmonary disease (COPD). | | Chemical Properties | White Solid | | Originator | Boehringer lngelheim (Germany) | | Uses | Anti - Asthmatic | | Uses | Specially selective choline drug resistance | | Uses | Muscarinic receptor antagonist. Bronchodilator | | Definition | ChEBI: An organic bromide salt having (1alpha,2beta,4beta,5alpha,7beta)-7-[(hydroxydi-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]non
ne as the counterion. Used (in the form of the hydrate) for maintenance treatment of airflow obstruction in patients with chronic obstructive pulmonary disease. | | Brand name | Spiriva | | General Description | Tiotropium bromide, (1 ,2 ,4 ,7 )-7-[(hydroxidi-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]nonane, (Spiriva) is anantimuscarinic agent that is used in an inhalation device to deliverthe drug into the lungs. It is indicated in the treatment ofchronic obstructive pulmonary disease (COPD), includingchronic bronchitis and emphysema. The standard once-dailydose is 18 g of tiotropium. | | Pharmacokinetics | Tiotropium is administered as a dry powder via inhalation using a
HandiHaler, in which is placed the drug, contained in a green capsule. Patients should be
cautioned not to be confused and take the medication orally. Systemic distribution following oral
inhalation is minimal, essentially because of its hydrophilic character. If swallowed, only
approximately 14% of the dose is eliminated in the urine, with the remainder being found in the
feces. Inhaled tiotropium has a 30-minute onset of action but a much longer duration of action
than ipratropium (24 versus <4 hours, respectively). Tiotropium is metabolized by both CYP3A4
and CYP2D6, followed by glutathione conjugation to a variety of metabolites. Only a very small amount is nonenzymatically hydrolyzed to inactive products. | | Clinical Use | Tiotropium is the dithienyl derivative of N-methyl scopolamine, a quaternary analogue of naturally
occurring scopolamine in Atropa belladonna. It is indicated primarily for the relief of
bronchospasms associated with COPD and can be considered to be a site-specific, local
medication to the lung. | | Side effects | Tiotropium has an adverse reaction
profile similar to that of ipratropium, with dry mouth being the most common adverse effect;
however, blurred vision, tachycardia, urinary difficulty, headache precipitation, and exacerbation
of narrow-angle glaucoma have been reported. | | Synthesis | At least two synthetic paths have been
disclosed in the patent and literature. The synthesis
of tiotropium is depicted in the scheme. Tropenol
hydrochloride 209 was first neutralized with ammonia in
toluene and then the free base was reacted with methyl di-(2-
thienyl)glycolate (210) in the presence of sodium hydride to
furnish desired tropenol ester 211 in 83% yield. The
vanadium-catalyzed oxidation of tropenol ester 211 using
hydrogen peroxide-urea complex gave epoxide 212, which
was converted into its quaternary salt 25 with methyl
bromide. The last two steps were carried out in a one-pot
process in 88%yield. 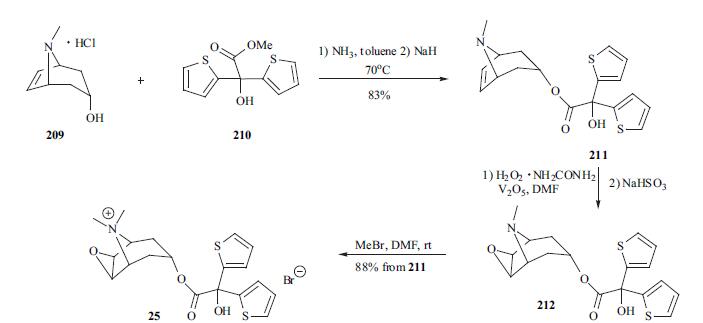
| | storage | Store at +4°C |
| | Tiotropium bromide Preparation Products And Raw materials |
|



