|
| Product Name: | Paxlovid | | Synonyms: | PF-07321332;PF-07321332-005;Nirmatrelvir-product under research;Unii-7R9A5P7H32;3-Azabicyclo[3.1.0]hexane-2-carboxamide,N- [(1S)-1-cyano-2-[(3S)-2-oxo-3-pyrrolidinyl]ethyl]- 3-[(2S)-3,3-dimethyl-1-oxo-2-[(2,2,2- trifluoroacetyl)amino]butyl]-6,6-dimethyl-, (1R,2S,5S)-;PF07321332(Paxlovid);nirmatrelvir;Nirmatrelvir (PF-07321332) | | CAS: | 2628280-40-8 | | MF: | C23H32F3N5O4 | | MW: | 499.53 | | EINECS: | 605-001-5 | | Product Categories: | 1;API;paxlovid;2628280-40-8 | | Mol File: | 2628280-40-8.mol |  |
| | Paxlovid Chemical Properties |
| Boiling point | 742.4±60.0 °C(Predicted) | | density | 1.267±0.06 g/cm3(Predicted) | | storage temp. | Store at -20°C | | solubility | DMSO : 140 mg/mL (280.26 mM; Need ultrasonic) | | form | A solid | | pka | 10.26±0.46(Predicted) | | InChIKey | LIENCHBZNNMNKG-OJFNHCPVSA-N | | SMILES | [C@@]12([H])[C@@]([H])(C1(C)C)CN(C(=O)[C@@H](NC(C(F)(F)F)=O)C(C)(C)C)[C@@H]2C(N[C@H](C#N)C[C@@H]1CCNC1=O)=O |
| | Paxlovid Usage And Synthesis |
| Synthesis | In
a 2 L three-necked flask, add 36.4 g (0.1 mol) of solid material SM1,
dissolve with 360ml butanone, then add 19.9 g Intermediate C (0.105
mol), 8.3g HOPO (0.075 mol) and stir for 10min, then add 38.7 g DIPEA
(0.3 mol), stirred for 10min, then added 26.6 g EDCI (0.15 mol), reacted
at 20-25![]() °C for 16-18 h, the raw material point basically
disappeared in TLC, and phosphoric acid aqueous solution (85%, 0.15 mol,
47.5 g was added to the reaction solution) ) and 360 ml of saturated
sodium chloride solution, stirred for 30 min, left to stand for
stratification, let go of the lower water layer, add 360 ml of sodium
chloride solution and 360 ml of isopropyl acetate solution to the
organic layer, extract and separate liquids, and concentrate the organic
layer to obtain 45.6 g of crude product, the yield is 91.3%. °C for 16-18 h, the raw material point basically
disappeared in TLC, and phosphoric acid aqueous solution (85%, 0.15 mol,
47.5 g was added to the reaction solution) ) and 360 ml of saturated
sodium chloride solution, stirred for 30 min, left to stand for
stratification, let go of the lower water layer, add 360 ml of sodium
chloride solution and 360 ml of isopropyl acetate solution to the
organic layer, extract and separate liquids, and concentrate the organic
layer to obtain 45.6 g of crude product, the yield is 91.3%.
In the
there-necked flask of 2 L, add crude product (50 g, 0.1 mol), 500 ml
isopropyl acetate, be heated to 65-70°C after solids are all dissolved,
under this temperature condition, add the n-heptane of 750 ml , heat
preservation and stirring 6-7 h, separate out a large amount of solid,
be cooled to room temperature, filter, filter cake is washed with the
organic solvent F of 300 ml (wherein, organic solvent F is that
isopropyl acetate and n-heptane are mixed by volume ratio of 1:1 mixed
solution), and dried to obtain 46.2 g of pure product with a yield of
92.3%. 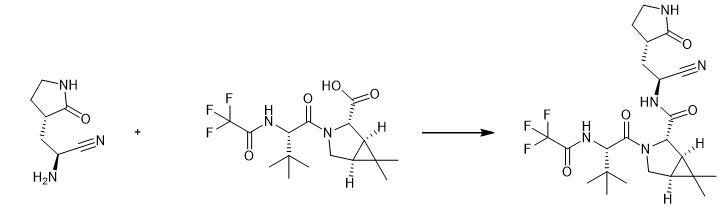 | | Description | PF-07321332 is an oral COVID-19 antiviral clinical candidate. By inhibiting the main protease, PF-07321332 prevents the virus from cleaving long protein chains into the parts it needs to reproduce itself. | | Uses | PF-07321332 (nirmatrelvir) is an oral antiviral clinical lead. It is a covalent inhibitor of SARS-CoV-2 Mpro (main protease, 3CLpro) that binds to cysteine145 within the enzyme's catalytic domain. Mpro is essential for coronavirus replication. Inhibiting Mpro actvity blocks replication at an early stage in the virus' life cycle. Due to structural similarities between the Mpro's of other coronavirusus, PF-07321332 offers the potential of pan-coronavirus activity. In vitro it is suggested to inhibit replication of CoV-2 variants including delta and omicron. | | Definition | ChEBI: Nirmatrelvir is an azabicyclohexane that is (1R,5S)-3-azabicyclo[3.1.0]hexane substituted by {(1S)-1-cyano-2-[(3S)-2-oxopyrrolidin-3-yl]ethyl}aminoacyl, 3-methyl-N-(trifluoroacetyl)-L-valinamide, methyl and methyl groups at positions 2S, 3, 6 and 6, respectively. It is the first orally administered inhibitor of SARS-CoV-2 main protease developed by Pfizer and used in combination with ritonavir for the treatment of COVID-19. It has a role as an EC 3.4.22.69 (SARS coronavirus main proteinase) inhibitor and an anticoronaviral agent. It is a nitrile, a member of pyrrolidin-2-ones, a secondary carboxamide, a pyrrolidinecarboxamide, a tertiary carboxamide, an organofluorine compound and an azabicyclohexane. | | Biological Activity | PF-07321332 is an orally bioavailable 3C-like protease (3CLPRO) inhibitor. This drug is being investigated for safety, tolerability, and pharmacokinetics before moving on to studies of efficacy in the treatment or prophylaxis of COVID-19. 3CLPRO is responsible for cleaving polyproteins 1a and 1ab of SARS-CoV-2. | | Structure and conformation | PF-07321332 is structurally similar to ML1000. PF-07321332's chemical structure was formally disclosed in Owen et al's Science article in November 2021, and this confirmed the structure that was obtained from the ACS meeting. |
| | Paxlovid Preparation Products And Raw materials |
|