| Description | β-Naphthyl methyl ether has an intensely sweet, floral odor suggestive of orange blossoms. It is free from naphthol by-odor. It has a sweet, strawberry taste. This may be prepared from potassium β-naphthol and methyl chloride at 300°C; by methylation of β-naphthol with dimethyl sulfate or by direct esterification with methyl alcohol.
|
| Chemical Properties | β-Naphthyl methyl ether has an intensely sweet, floral odor suggestive of orange blossoms; free from naphthol by-odor.
It has a sweet, strawberry taste |
| Chemical Properties | white powder |
| Chemical Properties | Methyl 2-Naphthyl Ether forms white crystals (mp
73–74°C) with an intense orange blossom odor. |
| Uses | 2-Methoxynaphthalene is an Impurity of the non-steroidal anti-inflammatory Naproxen (N377525). |
| Uses | 2-methoxynaphthalene acylation is used as a model reaction to study the catalytic benefits of delamination. It was also used to study the alkali-metal-mediated manganation (AMMMn) reactions. |
| Uses | 2-methoxynaphthalene acylation was used as a model reaction to study the catalytic benefits of delamination. It was also used to study the alkali-metal-mediated manganation (AMMMn) reactions. |
| Definition | ChEBI: 2-Methoxynaphthalene is a member of naphthalenes. |
| Preparation | From postassium β-naphthol and methyl chloride at 300°C; by methylation of β-naphthlol with dimethyl sulfate or by
direct esterification with methyl alcohol |
| Synthesis Reference(s) | Tetrahedron, 48, p. 6439, 1992 DOI: 10.1016/S0040-4020(01)88233-8
Tetrahedron Letters, 22, p. 3463, 1981 DOI: 10.1016/S0040-4039(01)81932-8 |
| Flammability and Explosibility | Notclassified |
| Synthesis | Preparation of 2-Methoxynaphthalene from 2-naphthol.
Principle: Phenols can be methylated to give methyl ethers. Methylation can be done either by using diazomethane or dimethyl sulphate in alkaline medium.
Reaction:
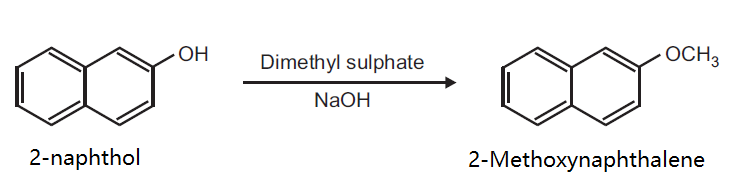
Procedure: Take 0.5 g 2-naphthol and 0.2 g NaOH in 5 ml distilled water in a beaker (25 ml). Heat on a wire gauze to obtain a clear solution. Cool the solution (10-15°C) and then add 0.35 ml dimethyl sulphate drop wise. After the addition is over, warm the mixture for one hour at 70-80°C and then cool. Filter the product and wash it with 10% sodium hydroxide solution and then with water. Dry the product, record the practical yield and re-crystallize it.
Re-crystallization: Dissolve the crude product in minimum amount of ethyl alcohol in a beaker by heating on a water bath. Filter the hot solution and cool the filtrate. Filter the white crystals of the product. Dry and record the melting point and TLC (using toluene as solvent).
|
| Purification Methods | Fractionally distil the ether under vacuum. Crystallise it from absolute EtOH, aqueous EtOH, *C6H6, pet ether or n-heptane, and dry it under vacuum in an Abderhalden pistol or distil it in vacuo. The picrate has m 118o (from EtOH or CHCl3). [Kikuchi et al. J Phys Chem 91 574 1987, Beilstein 6 III 2969, 6 IV 4257.] |



