|
| | 2-PHENYL-1,3-INDANDIONE Basic information |
| | 2-PHENYL-1,3-INDANDIONE Chemical Properties |
| Melting point | 144-148 °C(lit.) | | Boiling point | 243.3 °C (0.3 mmHg) | | density | 1.1404 (rough estimate) | | refractive index | 1.6600 (estimate) | | Fp | 208 °C | | storage temp. | Inert atmosphere,Room Temperature | | solubility | Very slightly soluble in water; slightly soluble in ethanol (96%) and in ether. Solutions are yellow to red. | | pka | pKa 4.09(H2O,t =25,I=0.1) (Uncertain) | | form | powder to crystal | | color | White to Light yellow | | Merck | 14,7234 | | CAS DataBase Reference | 83-12-5(CAS DataBase Reference) |
| | 2-PHENYL-1,3-INDANDIONE Usage And Synthesis |
| Description | Phenindione is an anticoagulant and vitamin K antagonist. It inhibits the reduction of vitamin K1 epoxide to vitamin K1 by vitamin K1 epoxide reductase in rat liver homogenates in a concentration-dependent manner. In vivo, phenindione (500 mg/kg) inhibits prothrombin synthesis and conversion of vitamin K1 epoxide to vitamin K1 in rats by 50 and 57%, respectively. Dietary administration of phenindione (4 mg/kg per day) inhibits aortic atherosclerosis or intracardial thrombosis in rat models of diet-induced atherosclerosis or intravascular thrombosis, respectively. | | Chemical Properties | light yellow fluffy fine flakes | | Uses | Oral anticoagulant (indanedione). | | Uses | Like coumarin derivatives, phenindione, a compound of the indandione class, acts by altering
biosynthesis of coagulant proteins in the liver. It is used for preventing and treating thrombosis,
thrombophlebitis, and thromboembolism. However, because of a number of side
effects such as polyurea, polydipsia, tachycardia, and others, it is rarely used in practical medicine. | | Definition | ChEBI: Phenindione is a beta-diketone and an aromatic ketone. It has a role as an anticoagulant. It derives from a hydride of an indane. | | Brand name | Hedulin (Sanofi Aventis). | | Clinical Use | Anticoagulant | | Synthesis | Phenindione, 3-phenylindan-1,3-dion (24.1.16), is synthesized in two ways.
The first consists of condensating benzaldehyde with phthalide in the presence of sodium
ethoxide. Evidently, the resulting phenylmethylenphthalide (24.1.15) rearranges under the
reaction conditions to give the desired phenindione (24.1.16). The second method consists of
condensation of phenylacetic acid with phthalic anhydride, forming phenylmethylenphthalide
(24.1.15), which rearranges further in the presence of sodium ethoxide to phenindione. 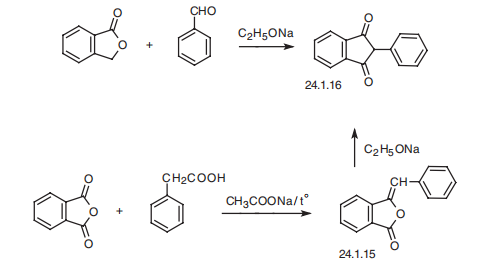
| | Drug interactions | Potentially hazardous interactions with other drugs There are many significant interactions with
coumarins. Prescribe with care with regard to the
following:
Anticoagulant effect enhanced by: alcohol,
amiodarone, anabolic steroids, aspirin, aztreonam,
bicalutamide, cephalosporins, chloramphenicol,
cimetidine, ciprofloxacin, dronedarone, fibrates,
clopidogrel, cranberry juice, danazol, dipyridamole,
disulfiram, fibrates, grapefruit juice, levofloxacin,
macrolides, metronidazole, nalidixic acid, neomycin,
norfloxacin, NSAIDs, ofloxacin, paracetamol,
penicillins, ritonavir, rosuvastatin, sulphonamides,
thyroid hormones, testosterone, tetracyclines,
tigecycline, tramadol, trimethoprim.
Anticoagulant effect decreased by: oral
contraceptives, rifamycins, vitamin K.
Anticoagulant effects enhanced/reduced by: anion
exchange resins, corticosteroids, dietary changes,
enteral feeds.
Analgesics: increased risk of bleeding with IV
diclofenac and ketorolac - avoid.
Anticoagulants: increased risk of haemorrhage with
apixaban, dabigatran, edoxaban and rivaroxaban -
avoid.
Ciclosporin: there have been a few reports of altered
anticoagulant effect; decreased ciclosporin levels have
been seen rarely | | Metabolism | Hepatically metabolised. Metabolites of phenindione
often colour the urine pink or orange. | | Purification Methods | Crystallise the dione from EtOH (m 156o) or CHCl3 (m 148-150o). [Beilstein 7 H 808, 7 III 4100, 7 IV 2570.] |
| | 2-PHENYL-1,3-INDANDIONE Preparation Products And Raw materials |
|