|
| | DI-TERT-BUTYLSILYL BIS(TRIFLUOROMETHANESULFONATE) Basic information |
| | DI-TERT-BUTYLSILYL BIS(TRIFLUOROMETHANESULFONATE) Chemical Properties |
| Boiling point | 73-75 °C/0.35 mmHg (lit.) | | density | 1.352 g/mL at 25 °C (lit.) | | refractive index | n20/D 1.398(lit.) | | Fp | 195 °F | | storage temp. | Inert atmosphere,Room Temperature | | solubility | sol most common organic solvents. | | form | Oil | | color | Clear Pale Yellow | | Specific Gravity | 1.358 | | Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents | | BRN | 3569211 | | Stability: | Moisture Sensitive | | InChIKey | HUHKPYLEVGCJTG-UHFFFAOYSA-N | | CAS DataBase Reference | 85272-31-7(CAS DataBase Reference) |
| Hazard Codes | C | | Risk Statements | 34 | | Safety Statements | 26-36/37/39-45 | | RIDADR | UN 3265 8/PG 2 | | WGK Germany | 3 | | F | 10-21 | | Hazard Note | Corrosive | | TSCA | No | | HazardClass | 8 | | PackingGroup | III | | HS Code | 29319090 |
| | DI-TERT-BUTYLSILYL BIS(TRIFLUOROMETHANESULFONATE) Usage And Synthesis |
| Chemical Properties | Colorless to yellow liquid | | Physical properties | bp 73–75°C/0.35 mmHg; d 1.208 g cm?3. | | Uses | Protecting group reagent for 1,3-diols used recently in a synthesis of N-homoceramides. Also used for selective α-galactosylation in the synthesis of α-galactosyl ceramides. | | Uses | Di-t-butylsilyl bis(trifluoromethanesulfonate)
is a reagent for the selective protection of polyhydroxy
compounds. This reagent reacts with 1,2-, 1,3-, and 1,4-diols
under mild conditions to give the corresponding dialkylsilylene
derivatives in high yield (0–50°C, 79–96%). Deprotection is
conveniently achieved by using aqueous hydrofluoric acid in acetonitrile
(eq 1).
Unlike di-t-butyldichlorosilane, this reagent reacts with hindered
alcohols. Even pinacol reacts to give the silylene derivative
(100°C, 24 h, 70%). Di-t-butylsilylene derivatives of 1,2-diols are
more reactive than those of 1,3- and 1,4-diols and undergo rapid
hydrolysis (5 min) in THF/H2O at pH 10, while the 1,3- and 1,4-
derivatives are unaffected at pH 4–10 (22°C) for several hours.
This protecting group is stable under the conditions of PDC oxidation
of alcohols (CH2Cl2, 25?C, 27 h) and tosylation of alcohols
(pyridine, 25°C, 27 h).
The reagent has seen limited use for the protection of alcohols
but has been used to protect nucleosides (eq 2).The procedure
consists of sequential addition of the ditriflate and triethylamine
to the nucleoside in DMF. The choice of solvent is critical.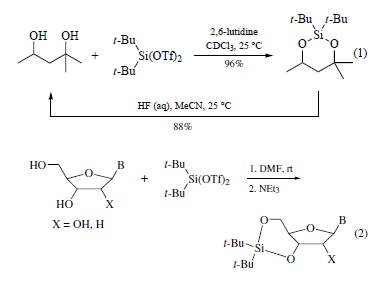 | | Preparation | by the treatment of di-t-butylchlorosilane
with trifluoromethanesulfonic acid, followed by distillation
(71% yield). |
| | DI-TERT-BUTYLSILYL BIS(TRIFLUOROMETHANESULFONATE) Preparation Products And Raw materials |
|