|
| | N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine Basic information | | Preparation |
| | N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine Chemical Properties |
| Boiling point | 76 °C0.3 mm Hg(lit.) | | density | 0.928 g/mL at 25 °C(lit.) | | refractive index | n20/D 1.492(lit.) | | Fp | 151 °F | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | Soluble in chloroform, ethyl acetate. | | pka | 7.29±0.50(Predicted) | | form | Liquid | | color | Clear colorless to light yellow | | Specific Gravity | 0.928 | | Sensitive | Moisture & Light Sensitive | | Hydrolytic Sensitivity | 2: reacts with aqueous acid | | BRN | 4311216 | | InChIKey | RPZAAFUKDPKTKP-UHFFFAOYSA-N | | CAS DataBase Reference | 93102-05-7(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-37/39 | | RIDADR | 1993 | | WGK Germany | 3 | | HazardClass | 3 | | PackingGroup | Ⅲ | | HS Code | 29319090 |
| | N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine Usage And Synthesis |
| Preparation | N-Methoxymethyl-N-(trimethylsilylmethyl)benzylamine is most conveniently prepared by treatment of benzylamine with chloromethyltrimethylsilane followed by formaldehyde and methanol. Access to higher ether homologs is achieved by replacing methanol with the appropriate alcohol. An alternate procedure involves alkylation of lithium N-benzyltrimethylsilymethylamide with methoxymethyl chloride.
| | Chemical Properties | N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine is clear colorless to light yellow liquid
| | Physical properties | bp 77–80°C/0.5 mmHg. | | Uses | N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine is useful reagent in synthesizing N-benzyl substituted pyrrolidines by [3+2] cycloaddition to α,ßunsaturated esters.
| | Uses | N-Benzyl-N-(methoxymethyl)-
N-trimethylsilylmethylamine (1) is a valuable reagent for in situ
generation of the N-benzyl azomethine ylide (2). It is generally
preferred over alternative silylmethylamine precursors6–8 because
of ease of handling and use. The ylide (2) is most conveniently
generated from (1) using a catalytic amount of trifluoroacetic acid
as described by Achiwa.Alternative catalysts include LiF,
TBAF,Me3SiOTf–CsF, or Me3SiI–CsF. Mechanistic studies
provide evidence that the reactive intermediate generated from
(1) with either CF3CO2H or F? is a 1,3-dipolar species. Reaction
of (2) with alkenes provides an efficient convergent route
to pyrrolidine derivatives. Alkynes afford 3-pyrrolines which can
be converted into pyrroles.The ylide (2) reacts most readily with
electron deficient alkenes and alkynes since this pairing results in
a narrow dipole HOMO–dipolarophile LUMO energy gap.Examples
of suitable dipolarophiles include unsaturated esters,
ketones, imides,nitriles,and sulfones. Cycloaddition occurs
with complete cis stereospecificity (eq 1) which is consistent
with a concerted mechanism. Dipolarophiles containing
an endocyclic double bond afford fused bicyclic pyrrolidines,
whereas substrates with an exocyclic double bond provide access
to spirocyclic systems.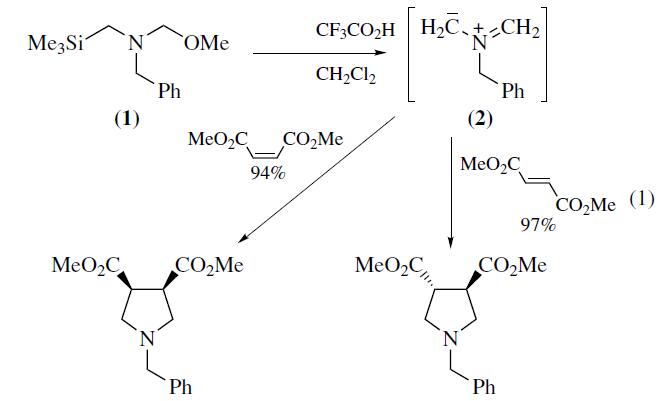 | | Uses | N-Methoxymethyl-N-(trimethylsilylmethyl)benzylamine forms azomethine ylides which readily undergo [3+2] cycloaddition to α,?-unsaturated esters affording N-benzyl substituted pyrrolidines in good yields. It reacts with asymmetric 1,3-dipolar cycloadditions in the practical, large-scale synthesis of chiral pyrrolidines. It is used in the the synthesis of 3-carboxy-1-azabicyclo[2.2.1]heptane derivatives, an important class of physiologically active compounds. | | Preparation | most conveniently prepared by treatment
of benzylamine with chloromethyltrimethylsilane followed
by formaldehyde and methanol.Access to higher
ether homologs is achieved by replacing methanol with
the appropriate alcohol. An alternate procedure involves alkylation of lithium N-benzyltrimethylsilymethylamide with
methoxymethyl chloride. |
| | N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine Preparation Products And Raw materials |
| Raw materials | Sodium hydroxide-->Ethyl acetate-->Formaldehyde-->Benzylamine-->Chloromethyltrimethylsilane | | Preparation Products | CIS-5-BENZYLTETRAHYDROPYRROLO[3,4-C]PYRROLE-1,3(2H,3AH)-DIONE-->1-Benzyl-3-trifluoromethyl-pyrrolidine-3-carboxylic acid-->Ethyl 1-benzyl-4,4-diMethylpyrrolidine-3-carboxylate-->3,4-Pyrrolidinedicarboxylic acid, 1-(phenylmethyl)-, 3,4-dimethyl ester, (3S,4S)--->2-BENZYL-HEXAHYDRO-CYCLOPENTA[C]PYRROL-4-ONE-->Methyl DL-1-benzyl-4-phenylpyrrolidine-3-carboxylate |
|



