|
| | Copper Chemical Properties |
| Melting point | 1083.4 °C (lit.) | | Boiling point | 2567 °C (lit.) | | density | 8.94 g/mL at 25 °C (lit.) | | vapor pressure | 0Pa at 20℃ | | Fp | -23 °C | | storage temp. | 2-8°C | | form | wire | | Specific Gravity | 8.92 | | color | Rust-brownish | | resistivity | 1.673 μΩ-cm, 20°C | | Water Solubility | insoluble | | Sensitive | air sensitive | | Merck | 13,2545 | | Exposure limits | TLV-TWA 1 mg(Cu)/m3 (dusts and mists)
(ACGIH and MSHA); 0.2 mg/m3 (fumes)
(ACGIH). | | Stability: | Stable. Incompatible with strong acids, active halogen compounds, chlorine, fluorine, iodine, bromine, ammonia. May react explosively with strong oxidizing agents. | | InChIKey | RYGMFSIKBFXOCR-UHFFFAOYSA-N | | CAS DataBase Reference | 7440-50-8(CAS DataBase Reference) | | NIST Chemistry Reference | Copper(7440-50-8) | | EPA Substance Registry System | Copper (7440-50-8) |
| | Copper Usage And Synthesis |
| Description | Copper has long been used by humans for a variety of reasons.
The name copper derives from the Latin for the metal, cuprum,
which is named for the Roman source, the island of Cyprus.
Copper has been used in a variety of alloys; of particular
importance among copper alloys is bronze, which comprised
most of the tools and weapons of the age that bears its name.
Brass, a copper–zinc alloy, is also highly used, for example, in
brass musical instruments. Copper has also long been used as
a building material, and owing to the metal’s malleability, as
well as high thermal and electric conductivity, continues to find
new uses. Copper and its compounds are naturally present in
the earth’s crust. Natural discharges to air and water may be
significant. Therefore, it is important to consider the background
levels that are commonly found and distinguish these
from high levels that may be found as a result of anthropogenic
activity. Copper is emitted into the air naturally from windblown
dust, volcanoes, and anthropogenic sources, the largest
of which are being primary copper smelters and ore processing
facilities. It is associated with particulate matter. The mean
concentration of copper in the atmosphere is 5–200 ng m-3. | | Chemical Properties | Reddish brown metal; face-centered cubic crystal; density 8.92 g/cm3; Mohs hardness 2.5 to 3.0; Brinnel hardness 43 (annealed); electrical resistivity 1.71 microhm-cm at 25°C; Poisson's ratio 0.33; melts at 1,083°C; vaporizes at 2,567°C; insoluble in water; dissolves in nitric acid and hot sulfuric acid; slightly soluble in hydrochloric acid; also soluble in ammonium hydroxide, ammonium carbonate and potassium cyanide solutions.
| | Chemical Properties | Copper is a reddish-brown metal which occurs free or in ores, such as malachite, cuprite, and chalcopyrite.
Copper is a group IB transition element on the periodic table and exists in four oxidation states: Cu0 Cu1+ (cuprous ion), Cu1+ (cupric ion), and Cu3+. In a natural state, copper is likely to be found in a variety of solid salts and compounds, but it can be found in the elemental form as well. Copper compounds generally are blue or green. The common green color of copper on exposure to air is a basic carbonate compound.

Copper is insoluble in water but readily dissolves in hot sulfuric and nitric acids. The vapor pressure is negligible at low temperatures, but in an industrial setting, in which very high temperatures are used to smelt copper ore, some potentially harmful copper fumes may be emitted. Although it not combustible in bulk, powdered copper may ignite. Fires and explosions may result from contact with oxidizing agents, strong mineral acids, alkali metals, and halogens (NIOSH, 2010).
| | Physical properties | Native copper has a distinctive reddish/brown color. Its first oxidation state (+1) formscompounds with copper ions named “cuprous,” also referred to as “copper(I),” and these ionsare easily oxidized with elements in group 16 (e.g., oxygen and sulfur) and elements in group17 (the halogens).Copper’s second oxidation state (+2) forms cupric compounds, also referred to as copper(II),which are more stable than copper(I) compounds. For example, copper in both oxidationstates can combine with fluorine: for copper(I) or cuprous fluoride, Cu+ + F- → CuF; and forcopper(II) or cupric fluoride, Cu2+ + 2F → CuF2.Copper’s melting point is 1,083°C, its boiling point is 2,567°C, and its density is 8.94g/cm3. | | Isotopes | There are 32 known isotopes of copper, ranging from Cu-52 to Cu-80. Only twoof these 32 isotopes of copper are stable, and together they make up the amount ofnatural copper found in the Earth’s crust in the following proportions: Cu-63 = 69.17%and Cu-65 = 30.83%. All the other isotopes of copper are radioactive and are artificiallyproduced with half-lives ranging from a few nanoseconds to about 61 hours. | | Origin of Name | Copper’s name comes from the Latin word cuprum or cyprium, which
is related to the name “Cyprus,” the island where it was found by the ancient Romans. | | Occurrence | Copper is the 26th most abundant element on Earth, but it is rare to find pure metallicdeposits. It is found in many different types of mineral ores, many of which are close to thesurface and easy to extract. It is found in two types of ores: (1) sulfide ores, such as covellite,chalcopyrite, bornite, chalcocite, and enargite; and (2) oxidized ores, such as tenorite, malachite, azurite, cuprite, chrysocolla, and brochanite.It is found in most countries of the world, but only a few high-grade deposits are costeffective to mine. Examples of some of its ores are cuprite (CuO2), tenorite (CuO), malachite[CuCO3 ? Cu(OH)2], chalcocite (Cu2S), covellite (CuS), bornite (Cu6FeS4), and chalcopyrite,also known as copper pyrite.Copper ores are found worldwide, in Russia, Chile, Canada, Zambia, and Zaire and, in theUnited States, in Arizona, Michigan, Montana, Nevada, New Mexico, Tennessee, and Utah.High-grade ores of 99% pure metal were found in the United States (and other countries), butmany of these native ore deposits have been mined over the past hundred years and are nowexhausted. Even so, many low-grade ores with concentrations of 10% to 80% pure copper stillexist and await a technology that will make them more profitable for exploitation. | | Characteristics | Copper, a versatile metal relatively easy to find, has made it useful for humans for manycenturies. It is malleable, ductile, and easily formed into many shapes such as ingots, pipes,wire, rods, tubing, sheets, powder, shot, and coins. Although copper is resistant to weak acids,it will dissolve in strong or hot acids. It resists atmospheric corrosion better than does iron.One reason is that it forms a bluish-green film (called patina) over its surface when exposed tomoist air or seawater. This coating of copper carbonate and copper sulfate provides a protective layer for the underlying metal that makes it ideal for use on boats, roofs, pipes, and coins.The surfaces of some copper church steeples and the Statue of Liberty have now oxidized toform a pleasing patina.One of copper’s most useful characteristics is that it is an excellent conductor of electricityand heat. | | History | The discovery of copper dates from
prehistoric times. It is said to have been mined for more than
5000 years. It is one of man’s most important metals. Copper
is reddish colored, takes on a bright metallic luster, and is malleable,
ductile, and a good conductor of heat and electricity
(second only to silver in electrical conductivity). The electrical
industry is one of the greatest users of copper. Copper occasionally
occurs native, and is found in many minerals such
as cuprite, malachite, azurite, chalcopyrite, and bornite. Large
copper ore deposits are found in the U.S., Chile, Zambia,
Zaire, Peru, and Canada. The most important copper ores
are the sulfides, oxides, and carbonates. From these, copper
is obtained by smelting, leaching, and by electrolysis. Its alloys,
brass and bronze, long used, are still very important; all
American coins are now copper alloys; monel and gun metals
also contain copper. The most important compounds are the
oxide and the sulfate, blue vitriol; the latter has wide use as an
agricultural poison and as an algicide in water purification.
Copper compounds such as Fehling’s solution are widely used
in analytical chemistry in tests for sugar. High-purity copper
(99.999 + %) is readily available commercially. The price of
commercial copper has fluctuated widely. The price of copper
in December 2001 was about $1.50/kg. Natural copper contains
two isotopes. Twenty-six other radioactive isotopes and
isomers are known. | | Uses | Copper is distributed widely in nature; it is the twenty-sixth most abundant element in the earth’s crust and is an essential element for many life forms. Copper is an abundant reddish, odorless metal that takes on a greenish-blue patina when exposed to the elements. It was the first metal worked by humans, and copper salts were among the first materials regularly used for therapeutic and cosmetic purposes.
Most animals require copper for certain biological processes. A deficiency of copper, as well as an excess, can have adverse health effects. The daily intake of copper in the United States ranges from 2 to 5 mg, almost all of which is excreted in the feces. Shellfish, seeds, nuts, and grains are rich sources of dietary copper. Minute amounts of cupric ion are absorbed and stored, mainly in the liver, blood, and brain. Copper is an essential cofactor in several enzyme systems (Shaligram and Campbell, 2012). Cuproenzymes catalyze important biochemical reactions, including iron absorption and hemebiosynthesis (Colotti et al., 2013).Copper deficiency may lead to anemia and neutropenia, and eventually to bone lesions resembling scurvy and to pathological fractures without hemorrhage (Kumar et al., 2005; Halfdanarson et al., 2008). Copper is also found in some intrauterine devices for the prevention of pregnancy (IPCS, 1998; Szymanski et al., 2012). | | Uses | Copper, being easy to mine and refine, has become a very versatile metal over the course ofcivilization. Early in human history, it was discovered that soft copper could be made harderand stronger when alloyed with other metals. Copper was and still is important to technologyand the development of civilizations. Over the past several thousand years, brass has foundmultiple uses, such as in coins, cooking utensils, and many types of instruments and hardwarethat are resistant to corrosion. Even today, brass is used to make musical instruments andbathroom, kitchen, and marine hardware. The U.S. one-cent penny was originally made ofcopper, but today the penny is made of zinc with a coating of copper. Copper is also an alloymetal used as a substitute for some of the silver in several other U.S. coins.Some common uses are in electrical wiring and components of electronic equipment,roofing, and pipes and plumbing and in the manufacturing of alloys such as brass, bronze,Monel metal, electroplating, jewelry, cooking utensils, insecticides, marine paints, cosmetics,and wood preservatives.Copper is second only to silver as an excellent conductor of electricity. This factor and itsavailability made it essential for the expansion of modern technologies. It was, and still is, adesired metal for wires to carry electricity, but the rapid expansion of modern communicationswould require more copper than could be made economically available. The solution has beento use optical fiberglass transmission cables as a substitute for copper wire. In addition, andeven more important, is the recent explosive growth of wireless transmission as a substitute forcopper wire in the communication industries. | | Uses | Copper is a metal that occurs naturally throughout the environment, in rocks, soil, water, and air. Copper is an essential element in plants and animals (including humans), which means it is necessary for us to live. Therefore, plants and animals must absorb some copper from eating, drinking, and breathing.
The use of copper dates back to prehistoric times. The metal, its compounds, and alloys have numerous applications in every sphere of life–making it one of the most important metals. Practically all coinages in the world are made out of copper or its alloys. Its alloys, bronze and brass, date from ancient times. More modern alloys such as monel, gun metals, and berylliumcopper also have wide applications. The metal is an excellent conductor of electricity and heat and is used in electric wiring, switches and electrodes. Other applications are in plumbing, piping, roofing, cooking utensils, construction materials, and electroplated protective coatings. Its compounds, namely the oxides, sulfates, and chlorides, have numerous of commercial applications.
Copper is distributed widely in nature as sulfides, oxides, arsenides, arsenosulfides, and carbonates. It occurs in the minerals cuprite, chalcopyrite, azurite, chalcocite, malachite and bornite. Most copper minerals are sulfides or oxides. Native copper contains the metal in uncombined form.The principal copper minerals with their chemical compositions and percentage of copper are listed below:
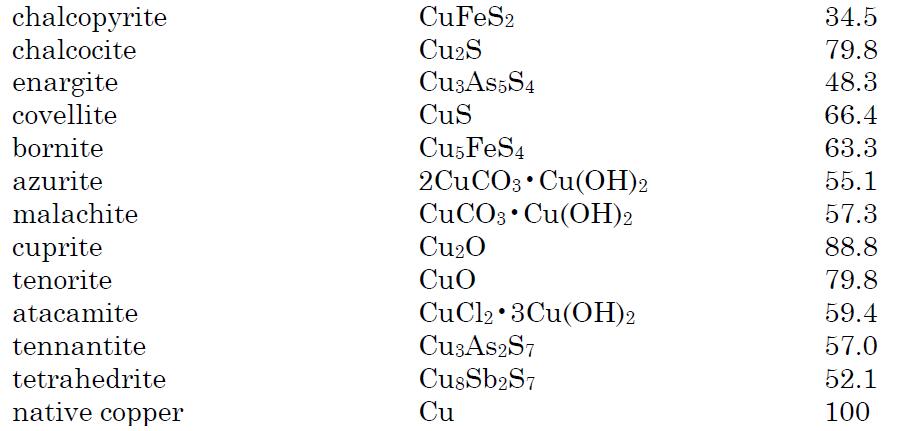 | | Production Methods | Copper can be found free in nature (although not as commonly
today). A naturally forming “patina” forms on copper
in the environment (e.g., Statue of Liberty in NewYork City).
The “patina” is commonly copper carbonate (from reaction
with water and carbon dioxide). This thin copper carbonate
layer covers exposed pure copper and prevents further oxidation.
The name copper is derived from the Latin word
cuprum, which, in turn, is derived from an earlier word,
cyprium or “Cyprium metal.” The Romans obtained much of
their copper from Cyprus, as the name implies.
Copper is found at a concentration of 50 ppm in the earth’s
crust, and its concentration in seawater is 0.001–0.02 ppm.
Although copper can also be obtained in an almost pure state
in nature, about 85% of the copper mined today is derived
from low-grade ores containing 2% or less of the metal. The
major ore is chalcopyrite (CuFeS2). Ores are removed by
open-pit mining as well as underground mining. | | Definition | Metallic element of atomic number 29, group IB of
the periodic table, aw 63.546, valences 1, 2; two
stable isotopes. | | General Description | Reddish lustrous malleable odorless metallic solid. | | Air & Water Reactions | Solid pieces are very slowly oxidized by air to give a green basic carbonate. Solid pieces become covered by a black oxide when heated in air. Insoluble in water. | | Reactivity Profile | Copper combines violently with chlorine trifluoride in the presence of carbon [Mellor 2, Supp. 1, 1956]. Is oxidized by sodium peroxide with incandescence [Mellor 2:490-93, 1946-1947]. Forms an unstable acetylide when acetylene is passed over samples that have been heated enough to form an oxide coating. Reacts more rapidly in powdered or granular form. Subject to explosive reaction then mixed in finely divided form with finely divided bromates chlorates and iodates of barium, calcium, magnesium, potassium, sodium, or zinc; these reactions are initiated by heat, percussion, and occasionally light friction [Mellor 2:310, 1946-1947]. A solution of sodium azide in Copper pipe with lead joints formed Copper azide and lead azide, both of these compounds can detonate [Klotz, 1973]. | | Hazard | Copper dust and powder, as well as a few of its compounds, are flammable, or even explosivewhen ignited in contained areas. Many of copper’s compounds are extremely toxic andpoisonous either with skin contact or when inhaled or ingested and should be handled by professionals in controlled environments. Even so, both plants and animals, including humans,require traces of copper for the proper metabolism of their foods. | | Health Hazard | Occupational workers exposed to copper fumes, dust and mists in work areas develop
symptoms of poisoning. These include irritation to the mucous membrane, nasal, and
pharyngeal irritation; nasal perforation, eye irritation, metallic or sweet taste, dermatitis;
prolonged periods of exposure to high concentrations cause anemia, adverse effects to the
lung, liver, and kidney. The exposed worker also suffers from metal fume fever; chills,
muscle aches, nausea, fever, dry throat, coughing, weakness, lassitude, irritation of the
eyes and the upper respiratory tract, discolored skin and hair, and acute lung damage.
Occupational workers exposed to copper dust suffer from gastrointestinal disturbances,
headache, vertigo, drowsiness, and hepatomegaly. Vineyard workers chronically exposed
to Bordeaux mixture (copper sulfate and lime) exhibit degenerative changes of the lungs
and liver. Dermal exposure to copper may cause contact dermatitis in some individuals. Copper is required for collagen formation. Copper defi ciency is associated with atherosclerosis and other cardiovascular conditions. Any kind of imbalance of copper in the
body causes health disorders that include, but are not limited to, arthritis, fatigue, adrenal burnout, insomnia, scoliosis, osteoporosis, heart disease, cancer, migraine headaches,
seizures, gum disease, tooth decay, skin and hair problems, and uterine fi broids, endometriosis (in females). Copper defi ciency is associated with aneurysms, gout, anemia, and
osteoporosis.
Exposures to copper in the form of dusts and mists cause irritation to the eyes, respiratory system, mucous membrane, nasal, pharyngeal irritation cough, dyspnea (breathing diffi culty), and wheezing. Prolonged exposures are known to cause nasal perforation.
Copper has caused anemia and damage to the lung, liver, and kidney in experimental
laboratory animals. Reports have indicated that copper dusts and fumes are potential
occupational carcinogens.
| | Agricultural Uses | Copper (Cu) is a transition element, belonging to Group 11 (formerly Group IA) of the Periodic Table. It is an important non-ferrous metal and a micronutrient with two distinct functions - to serve as a plant nutrient by being an activator or by being a part of certain enzymes like tyrosinase, lactase, ascorbic acid oxidase, cytochrome oxidase, etc., and secondly to play a role in many electron transfer processes.
adsorbed
Copper is a component of several enzyme complexes that influence carbohydrate and nitrogen metabolism in plants. Its other function is to neutralize harmful soil conditions. This is done by adding copper sulphate to the soil to maximize the crop yield. The addition of large quantities of copper in certain peat soils in Kerala (India) was found to precipitate or inactivate some toxins present in the soil and neutralize the harmful conditions. About 70% of copper in a plant is found in chlorophyll and plays an important metabolic function.
Plants absorb copper through leaves as cupric ions (Cu2+)i n the form of a complex, such as EDTA. In soils, copper is found mostly in the cupric (Cu2+) form, adsorbed by clay minerals as well as organic complexes to an extent of 2 to 100ppm. The content of soil organic matter, pH and other metallic ions such as iron, manganese or aluminum influence the availability of copper in the soil. The amount of exchangeable copper decreases as the pH increases. Enhancing the concentration of aluminum beyond 0.1 ppm in soil solutions is found to decrease copper uptake in wheat plants. The ratio of copper to other metallic ions in the rooting medium is more important for plant growth than the absolute concentration of copper.
The retention of copper in soil increases in proportion to the organic matter content. Depending on their stability, copper-humus complexes make copper available to plants. The copper content in soil ranges from 5 to 60mg per kg, although both lower and higher values are not uncommon. The average amount of copper in soils is about 9 to 10mg per kg. The most familiar copper mineral in soils, chalcopyrite (CuFeS2), has copper in the cuprous (Cu+) form. These minerals have very low solubility which increases with pH. The nature of the reactions of copper with various organic matter is still to be fully explored. Soils which are high in organic matter are more prone to copper deficiencies than those with a lesser organic matter content.
The symptoms of copper deficiency vary with crops and are similar to those of potassium deficiency. Copper has low soil mobility and is considered to be deficient when the copper level is below 4 ppm in dry matter. Copper deficiency causes the early aging of chlorophyll and a subsequent fall in crop yield. Lucerne, carrot, onion, barley, oats, wheat and orange are sensitive to copper deficiency. Copper deficiency is common in vegetables, small grains and fruits growing on sands, organic soils or over-limed acidic soils. In corn, the youngest leaves become yellow and stunted. As the deficiency becomes severe, the young leaves turn pale and the older ones die In many vegetable crops, the leaves lack turgor and assume a bluish-green hue. Stem melanosis is a disease occuring in certain beet varieties due to copper deficiency. Organic soils are often copper deficient, as are calcareous soils, the pH of which falls in the range of 8.0to 8.4. A foliar spray of a few kilograms of copper salt per hectare is enough to correct copper deficiency for many years. Copper deficiency increases the incidence of lodging, when simultaneous growth occurs as a response to nitrogen fertilization. Since pollen and ovaries are very sensitive to copper deficiency, flowering and fruiting may be adversely affected or even become absent.
Like most micronutrients, excess copper is toxic to plants. It reduces the iron activity and leads to iron deficiency.
Copper toxicity symptoms include a reduced shoot vigor, a poorly developed and discolored root system and leaf-chlorosis. The toxicity can be seen at places of iron ore deposits and copper smelting. In citrus and lettuce, high copper levels induce iron chlorosis. Copper also significantly inhibits the uptake of zinc, and vice versa.
The most common copper fertilizers include copper sulphate and copper ammonium phosphate. Copper sulphate solution is sprayed on plant leaves. Copper ammonium phosphate is added directly to the soil or sprayed on plants as a suspension in water. Copper salts, produced as frits or chelate, are suitable for soil application. Copper chelate are available for soil as well as for foliar application, in view of the slow release of copper to plants and prevention of copper ions getting converted into insoluble compounds in the soil. | | Industrial uses | The most important characteristics of copperimmersion coatings are their high electricalconductivity, good lubrication properties, andunique appearance. In addition to steel, they canbe applied to brass and aluminum and to printedcircuit boards.
Because of their conductivity, copper immersioncoatings have proved particularly usefulfor printed circuits. They are not especiallynoted for their decorative appeal, but can beused in applications where a particular appearanceis required, e.g., inexpensive, decorativehardware such as casket parts. Because of theirgood lubrication properties they can also beused on steel wire in die-forming operations. | | Safety Profile | Toxic by inhalation.
Questionable carcinogen with experimental
tumorigenic data. Experimental teratogenic
and reproductive effects. Human systemic
effects by ingestion: nausea and vomiting.
See also COPPER COMPOUNDS. Liquid
copper explodes on contact with water.
Potentially explosive reaction with acetylenic
compounds, 3-bromopropyne, ethylene
oxide, lead azide, and ammonium nitrate.
Iptes on contact with chlorine, chlorine
trifluoride, fluorine (above 121℃), and
hydrazinium nitrate (above 70'). Reacts
violently with C2H2, bromates, chlorates,
iodates, (Cl2 + OF2), dimethyl sulfoxide +
trichloroacetic acid, ethylene oxide, H202,
hydrazine mononitrate, hydrazoic acid, H2S
+ air, Pb(N3)2, K2O2, NaN3, Na2O2, sulfuric
acid. Incandescent reaction with potassium
dioxide. Incompatible with 1 -bromo-2
propyne. | | Potential Exposure | Exposure to fume may occur in copper and brass plants; and during the welding of copper alloys; Metallic copper is an excellent conductor of electricity and is widely used in the electrical industry in all gauges of wire for circuitry, coil, and armature windings; high conductivity tubes; commutator bars, etc. It is made into castings, sheets, rods, tubing, and wire and is used in water and gas piping; roofing materials; cooking utensils; chemical and pharmaceutical equipment and coinage. Copper forms many important alloys: Be-Cu alloy, brass, bronze; gunmetal, bell metal; German silver; aluminum bronze, silicon bronze; phosphor bronze; and manganese bronze. Copper compounds are used as insecticides, algicides, molluscicides, plant fungicides, mordants, pigments, catalysts; as a copper supplement for pastures; and in the manufacture of powdered bronze paint and percussion caps. They are also utilized in analytical reagents, in paints for ships’ bottoms; in electroplating; and in the solvent for cellulose in rayon manufacture. | | Environmental Fate | Copper reduces glutathione, which is necessary for normal cell
viability. The amino acid transferases are inhibited in the
presence of excess copper; lipid peroxidation also occurs.
Copper combines with thiol groups, which reduces the oxidation
state II to I in copper and oxidizes the thiol groups to
disulfides, especially in the cell membrane. | | Shipping | UN3089 Metal powders, flammable, n.o.s., Hazard Class: 4.1; Labels: 4.1-Flammable solid. Copper, elemental is not specifically cited in DOT’s PerformanceOriented Packaging Standards. | | Toxicity evaluation | The largest release of copper by far is to land, and the major
sources of release are mining and milling operations, agriculture,
solid waste, and sludge from publicly owned treatment
works. Sediment is an important sink and reservoir for copper. In relatively clean sediment, the copper concentration is <50 ppm; polluted sediment may contain several thousand ppm of copper.
Copper is released to water as a result of natural weathering of soil and discharges from industries and sewage treatment plants. Copper compounds may also be intentionally applied to water to kill algae. Of special concern is copper that gets into drinking water from the water distribution system.
The major species of soluble copper found in freshwater,
seawater, and a combination of the two over a range of pHs is
Cu2+, Cu(HCO3)+, and Cu(OH)2. At the pH values and
carbonate concentrations characteristic of natural waters, most
dissolved Cu(II) exists as carbonate complexes rather than as
free (hydrated) cupric ions.
The transport of copper is largely dependent on source
characteristics as well as particle size; however, it can bind to
many inorganic ligands. Some copper compounds are water
soluble, and this can increase transport distance, as well as
likelihood the metal will be taken up by organisms or adsorb to
organic residues. | | Incompatibilities | Copper dust, fume, and mists form shock-sensitive compounds with acetylene gas, acetylenic compounds, azides, and ethylene oxides. Incompatible with acids, chemically active metals, such as potassium; sodium, magnesium, zinc, zirconium, strong bases. Violent reaction, possibly explosive, if finely divided material come in contact with strong oxidizers | | Waste Disposal | Copper-containing wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill. Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill |
| | Copper Preparation Products And Raw materials |
| Raw materials | Sodium sulfite-->Copper(II) sulfate-->Copper(II) sulfate pentahydrate-->CHALCOPYRITE | | Preparation Products | (1,1-DIMETHYL-PROP-2-YNYL)-HYDRAZINE-->5-METHOXYBENZOFURAN-2-BORONIC ACID-->3-Bromotoluene-->lithium-ion battery-->Tolclofos-methyl-->COMBRETASTATIN A-4-->3-AMINO-6-METHOXYPYRIDAZINE-->5-Hydroxyanthranilic acid-->5-Methoxysalicylic acid-->4-CHLORO-2-PHENYLQUINAZOLINE-->2-Bromotoluene-->2-(2-AMINO-4-BIPHENYL)PROPIONITRILE-->2-PHENYL-4-[3H]QUINAZOLINONE-->TERT-BUTYL 4-(5-FORMYLPYRID-2-YL)PIPERAZINE-1-CARBOXYLATE-->9(10H)-ACRIDONE-->4-Ethylphenol-->1-(2-NITRO-BIPHENYL-4-YL)-ETHANONE-->4-Acetyl-2-fluorobiphenyl-->Pyriproxyfen-->N,N,N',N'-Tetraphenylbenzidine-->Diallyl maleate-->1-(2-amino[1,1'-biphenyl]-4-yl)ethan-1-one-->1,4-DINITROBENZENE-->SOLVENT YELLOW 85-->Vat Orange 11-->Cupric nitrate-->1,2-Dinitrobenzene-->Copper dinitrate-->Lobenzarit-->4-Iodophenoxyacetic acid-->Masoprocol-->1,2,3,4-Butanetetracarboxylic acid-->5-Hydroxyvanillin-->2-((4-ETHOXYPHENYL)AMINO)-4-NITROBENZOIC ACID-->4-Iodophenol-->2,4-DIMETHYLQUINOLINE-->3-Methylfuran-->FLAVANTHRONE-->Arsenic-copper alloy-->polyimide adhesive YJ-8 for strain gauge |
|