|
| Product Name: | Azilsartan | | Synonyms: | AZILSARTAN;2-Ethoxy-1-[[2'-(4,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl]benzimidazole-7-carboxylic acid Azilsartan;2-Ethoxy-1-{[2′-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylic acid;2-ethoxy -1- {[2'-(5-oxo-4,5-dihydro -1,2,4 -oxadiazol-3-yl) biphenyl-4-yl ] methyl} -1H –benzimi[d]dazole-7-carboxylic acid;2-ethoxy-1-((2'-(5-oxo-4,5-dihydro-
1,2,4-oxadiazol-3-yl)-[1,1'-biphenyl]-4-yl)methyl)-1H-benzo[d]imidazole-7-carboxylic acid;AZATMint-E;Azilsartan, ≥98% (HPLC);CS-865 | | CAS: | 147403-03-0 | | MF: | C25H20N4O5 | | MW: | 456.45 | | EINECS: | 808-058-6 | | Product Categories: | Intermediates;Aromatics;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;147403-03-0 | | Mol File: | 147403-03-0.mol |  |
| | Azilsartan Chemical Properties |
| Melting point | 188 °C(dec.) | | density | 1.42 | | storage temp. | Sealed in dry,2-8°C | | solubility | DMSO: soluble15mg/mL (clear solution) | | form | powder | | pka | 2.05±0.10(Predicted) | | color | white to beige | | InChIKey | KGSXMPPBFPAXLY-UHFFFAOYSA-N |
| | Azilsartan Usage And Synthesis |
| Description | Edarbi (azilsartan medoxomil), a prodrug, is hydrolyzed to azilsartan in the gastrointestinal tract during absorption. Azilsartan is a selective AT1 subtype angiotensin II receptor antagonist.
The drug substance used in the drug product formulation is the potassium salt of azilsartan medoxomil, also known by the US accepted name of azilsartan kamedoxomil and is chemically described as (5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-ethoxy-1-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylate monopotassium salt. | | Uses | Azilsartan and chlorthalidone combination is used to treat high blood pressure (hypertension). Azilsartan is an angiotensin II receptor blocker (ARB). It works by blocking a substance in the body that causes blood vessels to tighten. As a result, azilsartan relaxes the blood vessels. This lowers blood pressure and increases the supply of blood and oxygen to the heart. | | Pharmacokinetics | Azilsartan differs structurally from candesartan only by replacement of candesartan’s five-membered tetrazole ring with a five-membered oxo-oxadiazole ring. Azilsartan is formulated as an ester prodrug, azilsartan medoxomil, which is rapidly hydrolysed to the bioactive molecule during gastrointestinal absorption. It produces dose-dependent reductions in vascular smooth muscle contraction, peripheral resistance, and the synthesis and effects of aldosterone on the kidneys. Azilsartan has a half-life of about 11 hours and is metabolized in the liver mainly via CYP2C9 and is eliminated in both the urine and feces. | | Mechanism of Action | Edarbi, a prodrug, is hydrolyzed to azilsartan in the gastrointestinal tract during absorption. Azilsartan is a selective AT1 subtype angiotensin II receptor antagonist. Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzymes (ACE, kinase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Azilsartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is, therefore, independent of the pathway for angiotensin II synthesis. | | Biological Activity | Potent angiotensin II type 1 (AT1) receptor inverse agonist (IC50 = 2.6 nM at the human AT1 receptor). Inhibits angiotensin II-induced IP1 accumulation in COS-7 cells; decreases maximal contraction of rabbit aortic strips in a concentration-dependent manner (pD'2 = 9.9). Antihypertensive; prevents vascular cell proliferation and expression of PAI-1. | | Side Effects | Overall, azilsartan is well-tolerated in most patients in terms of adverse effects. In a controlled, double-blind, randomized clinical trial comparing the efficacy of azilsartan medoxomil to the ACE inhibitor ramipril in 784 patients, the overall number of adverse events was reported to be much less frequent with azilsartan.
During the treatment period, dizziness and hypotension occurred more often with the azilsartan treatment groups, and cough, a class side effect commonly encountered with ACE inhibitors, occurred more frequently with the ramipril group (8.2% of participants). Cough only occurred in 1% and 1.4% of azilsartan 40 and 80 mg groups, respectively. Another effect observed in the azilsartan group was a pertinent increase in serum potassium, sodium, and uric acid; no deaths resulted from this effect or the aforementioned adverse effects as well.
| | Description | Azilsartan is an antagonist of the angiotensin II type 1 receptor (AT1; IC50 = 0.42 μM) and the active metabolite of azilsartan medoxomil . Azilsartan is formed from azilsartan medoxomil by hydrolysis in the gastrointestinal tract and liver. Azilsartan also acts as an inverse agonist, inhibiting angiotensin II-induced accumulation of inositol-1-phosphate in COS-7 cells expressing recombinant human AT1 (IC50 = 2.6 nM). It reduces the maximal contractile response induced by angiotensin II in isolated rabbit aortic strips (pD2 = 9.9). Azilsartan (100 ng/kg, i.v.) inhibits the angiotensin II-induced pressor response in conscious normotensive rats. | | Chemical Properties | White to Off-White Solid | | Uses | Azilsartan is an analgesic and antiinflammatory drugs containing angiotensin II antagonists. | | Uses | Azilsartan is an angiotensin II type 1 (AT1) receptor antagonist with IC50 of 2.6 nM | | Definition | ChEBI: A benzimidazolecarboxylic acid that is benzimidazole-7-carboxylic acid substituted at position 2 by a methoxy group and at position 1 by a 2'-[(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl group. Used (as the prodrug, azilsartan medoxomil) f
r treatment of hypertension. | | Clinical Use | Azilsartan is an orally active angiotensin II blocker which was approved and launched in Japan for
the treatment of arterial hypertension in May 2012. Azilsartan, which is marketed under the trade
name Azilva®, was discovered and developed by Takeda—the same firm which had developed and
launched a prodrug of azilsartan (azilsartan kamedoxomil, Edarbi®) in 2010. Azilsartan exhibits higher
potency and slower off-rate kinetics for type 1 angiotensin II receptors, which contributes to azilsartan’s
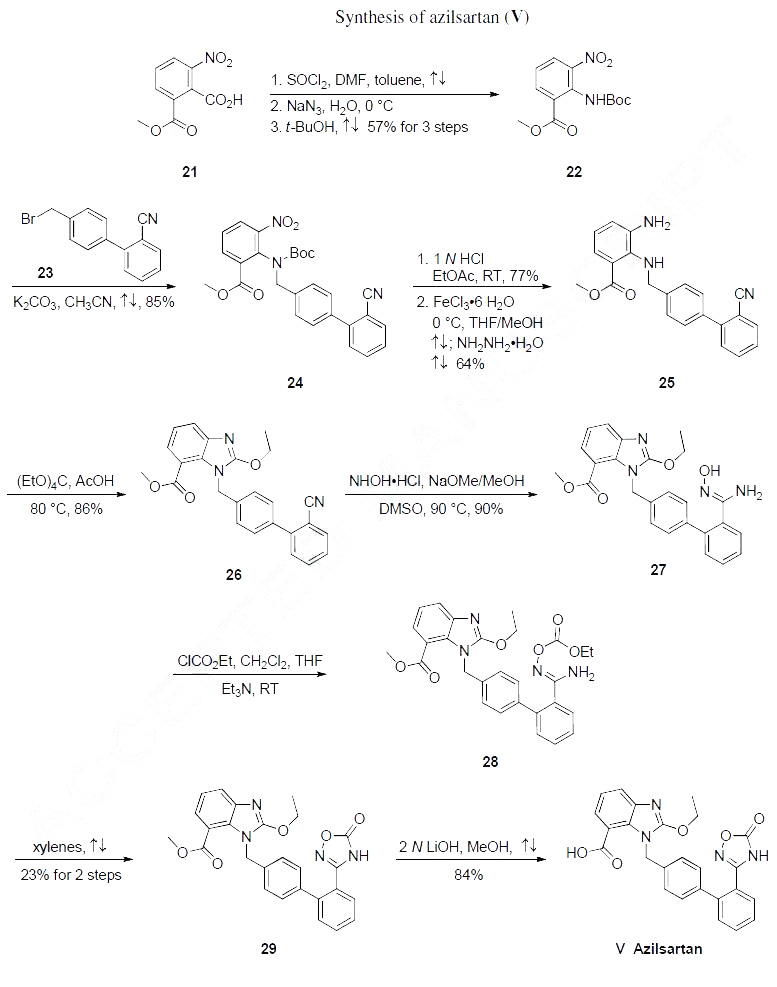
comparatively improved blood pressure lowering effect. | | Synthesis | The most likely process-scale synthetic route likely mimics that which is disclosed in Takeda?ˉs
patents, and this is described in the scheme below. Commercial available benzoic acid 21 was activated as
the correspndong acyl azide and underwent a Curtius rearrangement to give carbamate 22 in 57% yield
(three steps from compound 21). The resulting aniline 22 was then alkylated with commercial 4-
(bromomethyl)-2'-cyanobiphenyl 23 to give benzylamine 24 in 85% yield. Nitroamine 24 was then
exposed to mildly acidic conditions to affect Boc-removal prior to reduction via ferric chloride hydrate
in the presence of hydrazine hydrate. The resulting diamine 25 arose in 64% yield across the two-step
sequence. Interestingly, tt was found that metal catalysts under conventional hydrogenation conditions
caused partial debenzylation, which led the authors to arrive at the hydrazine/ferric chloride conditions.
Next, benzimidazole formation was achieved upon treatment of diamine 25 with ethyl orthocarbonate in
acetic acid. The resulting ethoxylbenzimidazole 26 was procured in 86% yield, and this benzonitrile was
further reacted with hydroxylamine hydrochloride and sodium methoxide to provide amidoxime 27 in
90% as white powder. Next, activation with ethyl chlorocarbonate gave 28 followed by heating in
refluxing xylene to give oxadiazolone 29 in 23% yield from hydroxyamidine 27. Finally ester 29 was
saponified with 2N LiOH in methanol to give azilsartan (V) in 84% yield.

An improved scalable route (Scheme below) to azilsartan was reported and features reproducibly better
yields.43 Hydroxyamidine 30 was treated with dimethyl carbonate and sodium methoxide, which
triggered they key cyclization along with concomitant transesterification to deliver 29. Milder aqueous
sodium hydroxide hydrolysis converted this methyl ester 29 to azilsartan (V) in 88-90% yield. |
| | Azilsartan Preparation Products And Raw materials |
|




