| Description | There are approximately 400 million people worldwide
with chronic hepatitis B virus (HBV) infection, about onethird
of whom have potentially progressive and life-threatening
liver disease associated with the infection. Chronic
hepatitis B infection can lead to cirrhosis, liver failure and hepatocellular carcinoma. Globally, HBV infection accounts
for over one million deaths annually. At present, lamivudine
and adefovir dipivoxil are the only approved nucleoside/
nucleotide analogs for the treatment of HBV infection. However,
resistance to lamivudine is now recognized in 16 to
32% of HBV-infected patients after the first year of monotherapy
and about 50% of patients after two years.
With adefovir treatment, the resistance rate is much lower, at
about 2.5% after two years of therapy. Experience in treating
chronic HIV infections has proven the advantage of therapy
with a combination of antiviral compounds. Similarly for
HBV, there is a clear need for additional antiviral compounds.
Several promising candidates are currently in clinical
development. Idenix (then known as Novirio) discovered
that the known beta-L-nucleosides, L-dA, L-dC (torcitabine)
and L-dT (telbivudine), have highly specific activity against
HBV. These L-nucleosides are essentially without activity
against any of the other viruses tested and are similarly
without effect in cell culture and in vivo toxicological tests.
However, they are phosphorylated within human cells to
their triphosphates which inhibit the HBV DNA polymerase,but not human polymerases. Of these three compounds,
telbivudine was the only one to combine reasonable
oral bioavailability with good anti-HBV activity and so was
progressed to development jointly with Novartis with the
highest priority. |
| Uses | Antiviral (treatment of
hepatitis B infection). |
| Brand name | (Novirio). |
| Acquired resistance | After 1 year resistance occurred in 7–20% of patients on telbivudine
depending upon past exposure to other drugs used
in the treatment of hepatitis B and the type of infection.
Development of resistance was less frequent in those receiving
telbivudine than in those receiving lamivudine. |
| Pharmaceutical Applications | A synthetic thymidine nucleoside analog formulated for oral
use in the treatment of chronic hepatitis B infection. |
| Pharmacokinetics | Oral absorption: 100%
Cmax 600 mg/kg oral: 3.7 μg/mL
Volume of distribution: In excess of body water
Plasma protein binding:3.3%
It is eliminated renally, necessitating dose adjustment in
patients with renal insufficiency.
It should not be administered with pegylated interferon
because of an increased risk of neuropathy. |
| Clinical Use | Treatment of chronic hepatitis B in patients >16 years of age |
| Side effects | Adverse effects are similar to those of lamivudine and include
upper respiratory tract infection, headache, fatigue and gastrointestinal
upset. Myopathy and peripheral neuropathy are
rare but have been observed in some patients several weeks
into the course with associated rise in serum creatine kinase
levels. Acute exacerbations of hepatitis have been observed on
discontinuation of therapy. Lactic acidosis may occur, necessitating
drug discontinuation. |
| Synthesis | The synthesis of telbivudeine is depicted in the scheme. The L-arabinose (105) was treated with acid in
methanol to form the semi-acetyl intermediate which was
then reacted with benzoyl chloride to provide 106 in 50%
yield. Acetolysis of 106 with a mixture of acetic acid
and acetic anhydride afforded 107 in 95% yield. The α/β
mixture was directly condensed with activated thymine to
give 108. The nucleoside 108 was purified by column chromatography
and characterized as the α-anomer. Debenzoylation
of 108 with sodium methoxide in methanol afforded
109. Differentiation of the 2’-OH was achieved by selective
protection of the two other hydroxyl groups with 1,3-
dichloro-1,1,3,3,-tetraisopropyldisiloxane to form 110. In
order to limit undesired reaction during the deoxygenation
step, 110 was transformed into o-phenylthiocarbonate 111 which upon treatment with tributyltin hydride under Barton’s
conditions afforded 112 in good yield. Desilylation of 112
gave Telbivudine (XIV). 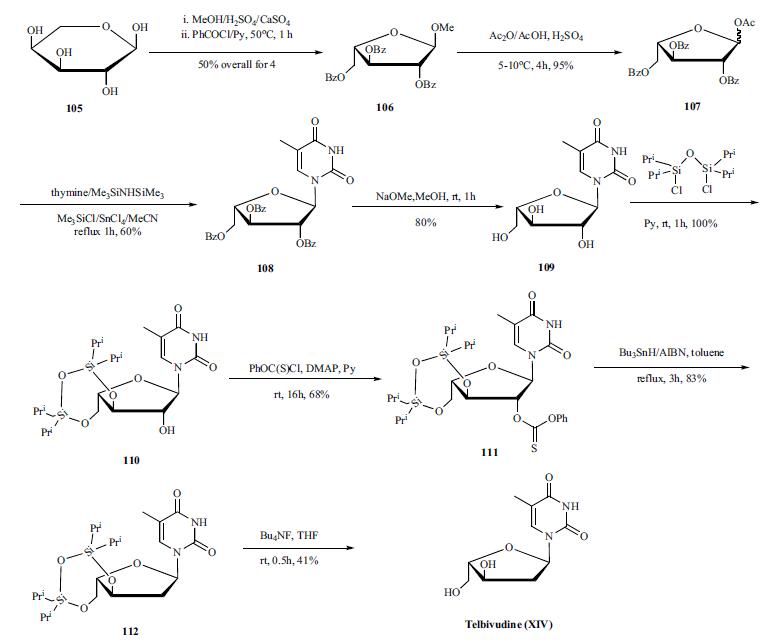
|
| Drug interactions | Potentially hazardous interactions with other drugs
Interferons: increased risk of peripheral neuropathy. |
| Metabolism | Telbivudine is not metabolised. It is eliminated primarily
by urinary excretion of unchanged substance. |