|
| | Glibenclamide Chemical Properties |
| Hazard Codes | Xn | | Risk Statements | 20/21/22 | | Safety Statements | 22-36/37/39-36-24/25 | | WGK Germany | 2 | | RTECS | YS4725200 | | HS Code | 29350090 | | Toxicity | LD50 in rats and mice (g/kg): >20 orally; >12.5 i.p.; >20 s.c. (Mizukami) |
| | Glibenclamide Usage And Synthesis |
| Hypoglycemic agents | Glibenclamide belongs to the second generation oral sulfonylurea drugs with the mechanism of action being similar as tolbutamide and the hypoglycemic effect being strongest among sulfonylurea drugs. Its intensity of action is about 200 to 250 times of that of Tolbutamide. It can selectively act on the pancreatic β-cells, promote insulin secretion; can enhance the hypoglycemic effect of exogenous insulin and strengthen the post-receptor effect of insulin. It has fast oral absorption with high protein binding rate. It begins to take effect after 30 minutes with the effect being strongest at 1.5 hours and the duration of 16 to 24 hours. It has a distribution volume of 0.1L/kg, plasma protein binding rate of 90% to 95% and half-life of 4 to 8 hours. It is mainly consumed by the liver metabolism with six metabolites. Two of them are known as hydroxylated compounds with no hypoglycemic effect and is mainly excreted from the urine and a small amount is excreted by the stool. It is clinically mainly used for the treatment of mild to moderate non-insulin dependent diabetes mellitus.
Recently, an international study found that the commonly used diabetes drug glibenclamide can help the body's immune system to fight against certain bacterial infections, e.g. in the treatment of melioidosis, the mortality rate can be reduced by about half.
Melioidosis is a disease prevalent in tropical areas such as Southeast Asia and northern Australia. It is caused by Burkholderia pseudomallei with the symptoms including sepsis and pneumonia. The mortality rate is high. Diabetic patients tend to be more susceptible to melioidosis, but the mortality rate is lower compared with other patients. | | Hypoglycemic effect | Glibenclamide is currently one of the most commonly used drugs for the treatment of type 2 diabetes. Because this product has fast and strong effect, so the effect after the application remarkable, being able to have good control of blood sugar; being applicable for patients of high blood sugar who get bad efficacy when treated with other sulfonyl Urea hypoglycemic agents.
Two commonly sulfonylurea oral hypoglycemic agents are glibenclamide and glimepiride, the comparison of hypoglycemic effect of them two are as follow:
(1) The effect on glucose transport and metabolism: Glibenclamide and glimepiride can stimulate the key enzymes in the glucose metabolism and improve the glucose transporter (GLUT4) translocation/dephosphorylation to promote the glucose uptake of the surrounding tissue, specifically exhibited in glycogen synthesis and increased fat formation. Glycogen synthase and 3-phosphoglycerol fatty acyltransferase are the key enzymes in glycogen and fat synthesis, and glimepiride is more active than glibenclamide in activating these key enzymes, such as for activating glycogen synthase activity, glimepiride is 2.5 times that of glibenclamide; for the capability to activate lipase, glimepiride is 1.9 times that of glibenclamide. Glimepiride increased the expression of GLUT4 on the cell membrane by inducing dephosphorylation of GLUT4.
(2) Effect on glycosylated-phosphatidylinositol-specific phospholipase (GPI-PLC): GPI is located in the outer layer of the cell membrane and participates in insulin signal transduction, which can interfere with glucose metabolism of muscle and adipocytes. GPI-PLC can shed the GPI, thereby improving the cell phosphorylation status. Insulin and sulfonylureas can activate GPI-PLC, helping muscle, adipose tissue for the uptake and transport of glucose. However, in the presence of insulin resistance, insulin itself is very difficult to activate GPI-PLC, but glimepiride can still activate the enzyme. In vitro and in vivo studies have shown that, glimepiride has the strongest pancreatic effect in sulfonylurea drugs, which can increase glucose synthesis by 2.5 times and fat synthesis by 4 times. The ratio of glimepiride to glibenclamide was 2: 1. Therefore, glimepiride has a lower secondary failure incidence than other sulfonylurea drugs. | | Precautions | Glibenclamide should be started at low doses. During treatment, it should be regularly checked of the urine sugar, urine ketone body, urine protein and blood sugar, blood routine examination, liver and kidney function, vision, retinal blood vessels.
(1) Patients of liver and kidney dysfunction, leukopenia, sulfa allergy, pregnant women and diabetes complicated by acidosis and acute infection should be disabled.
(2) It can cause abdominal distension, abdominal pain, anorexia, nausea and other gastrointestinal reactions. It can also appear as allergy (skin erythema or urticaria), leukopenia, granulocyte deficiency, thrombocytopenia, hypoglycemia, etc., should be immediately discontinued and treated. Among them, hypoglycemia reaction is more common.
(3) Glibenclamide and other sulfonylurea hypoglycemic agents can’t be combined with thiazide diuretics or glucocorticoids.
(4) It should be avoided to taken together with anticoagulant drugs such as dicoumaroline.
(5) When combined with phenylbutazone, the hypoglycemic effect can be enhanced, causing acute hypoglycemia.
(6) Combination with sulfonamides can enhance both the effect and toxicity of glibenclamide, not suitable for use.
(7) Combination with chloramphenicol can also enhance the effect and toxicity of this product; combination of two drugs demands dose adjustment according to the patient's blood sugar levels, otherwise can cause hypoglycemic shock.
(8) Sulfonylurea drugs can enhance the toxicity of alcohol; alcohol should be avoided during treatment. | | Chemical properties | It appears as white crystalline powder. M.p. 168-173 ° C. It is insoluble in water, slightly soluble in ethanol, acetone and chloroform. | | Uses | Hypoglycemic agents with stronger effect than toluene sulfonylurea; used for the treatment of mild, non-insulin-dependent diabetes; | | Hazards & Safety Information | Category : Toxic substances
Toxic classification: poisoning
Acute toxicity: Intraperitoneal-rat LD50: 3750 mg/kg; Oral-mouse LD50: 3250 mg/kg
Flammability Hazardous characteristics : Thermal decomposition releases toxic nitrogen oxides, sulfur oxides, chloride fumes
Storage and transport characteristics: Treasury: low temperature, ventilated and dry
Extinguishing agent : water, carbon dioxide, foam, dry powder | | Description | Glyburide (10238-21-8) is a second generation oral hypoglycemic agent. Acts via ATP-dependent K+ channel (Kir6, KATP) block.1?Inhibits Kir6 currents in the pancreas, causing an increase in intracellular Ca2+ and insulin secretion. Glyburide also inhibits recombinant CFTR Cl- channels with an IC50 of 20 μM.2?Cell permeable. | | Chemical Properties | White or almost white, crystalline powder | | Originator | Daonil,Hoechst,Germany | | Uses | An ATP-dependent KIR6 and CFTR Cl- channel blocker | | Uses | antihyperglycemic | | Uses | Glyburide is a second generation sulfonylurea with hypoglycemic activity. Glyburide is an antidiabetic. | | Uses | Glyburide (Glibenclamide) is a sulfonylurea compound that modulates insulin production. Sulfonylureas bind to ATP-dependent K+ channels in beta cells of the pancreas, depolarizing them and stimulating the release of Ca2+, which in turn stimulates insulin production. Glyburide (Glibenclamide) is an ATP-dependent K+ channel (KIR6, KATP) and CFTR Cl- channel blocker. This compound inhibits recombinant CFTR Cl- channels (IC50 = 20 ?M). | | Definition | ChEBI: An N-sulfonylurea that is acetohexamide in which the acetyl group is replaced by a 2-(5-chloro-2-methoxybenzamido)ethyl group. | | Manufacturing Process | To a solution of 10.2 g of 4-(β-(2-ethoxy-5-chlorobenzamido)ethyl)-
benzenesulfonamide in 12.5 ml of 2 N sodium hydroxide solution and 30 ml
acetone are added dropwise, at 0-5°C, 3.3 g of cyclohexyl isocyanate. The
whole is stirred for 3 hours, diluted with water and methanol, undissolved
matter is separeted by filtration. The filtrate is acidified with dilute
hydrochloric acid. The 4-(β-(2-ethoxy-5-chlorobenzamido)ethyl)-
benzenesulfonyl)-N'-cyclohexylurea which precipitates in the form of crystals
melts after recrystallization from methanol at 168-170°C. | | Brand name | Micronase (Pharmacia & Upjohn). | | Therapeutic Function | Oral hypoglycemic | | General Description | Similar to glipizide, glyburide, 1-[[p-[2-(5-chloro-o-anisamido)ethyl]-phenyl]sulfonyl]-3-cyclohexylurea(DiaBeta, Micronase, Glynase), is a second-generationoral hypoglycemic agent. The drug has a half-life eliminationof 10 hours, but its hypoglycemic effect remains for upto 24 hours. | | General Description | Glyburide (glibenclamide) is 5-chloro-N-[2-[4-[[[(cyclohexylamino)carbonyl]amino]sulfonyl]phenyl]ethyl]-2-methoxybenzamide; this compound can also be named asthe urea—see preceding discussion (Diabeta, Glynase,generic). Some tablet formulations contain micronized drug(formerly Micronase, now only generic). Combinations areavailable with metformin in the United States (Glucovance,generic; tablets, mg glipizide/mg metformin as hydrochloride:1.25/250, 2.5/500, 5/500). | | Biological Activity | ATP-dependent K + channel (K ATP ) and CFTR Cl - channel blocker. Inhibits K ATP currents in the pancreas, causing an increase in intracellular Ca 2+ and insulin secretion. Inhibits recombinant CFTR Cl- channels with an IC 50 of 20 μ M. | | Biochem/physiol Actions | Selectively blocks ATP-sensitive K+ channels; high affinity binding sites found in brain, pancreatic β cells, and cardiovascular system. | | Mechanism of action | This drug belongs to the second-generation sulfonylurea derivatives. Like all of the other
oral hypoglycemic drugs examined, it is a β-cell stimulant in pancreas; but on the other
hand, it increases the sensitivity to insulin, the degree to which it binds with target cells.
At the same time, it differs in that it is easier to tolerate. The hypoglycemic effect sets in at significantly lower doses than with first-generation drugs. | | Clinical Use | Non-insulin dependent diabetes mellitus | | Synthesis | Glyburide, 1-[4-[2-(5-chloro-2-methoxybenzamido)ethyl]-phenylsulfonyl]-3-
cyclohexylurea (26.2.11), is a second-generation drug that differs from those described above
in that it has a more complex structure in the sulfonylamide region of the molecule into which
an additional pharmacophore group is added. It is synthesized from 2-methoxy-5-chlorobenzoic
acid chloride, which is transformed into an amide 26.2.9 by reacting it with 2-phenylethylamine.
This undergoes subsequent sulfonylchlorination by chlorosulfonic acid, and then
amination by ammonia, which gives sulfonamide 26.2.10. The resulting sulfonamide is
reacted with cylclohexylisocyanate to give the desired glyburide (26.2.11). 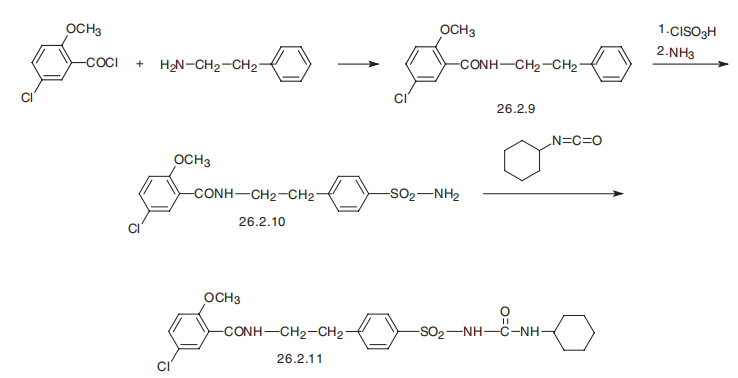
| | Drug interactions | Potentially hazardous interactions with other drugs
Analgesics: effects enhanced by NSAIDs.
Antibacterials: effects enhanced by chloramphenicol,
sulphonamides, tetracyclines and trimethoprim;
effects possibly enhanced by ciprofloxacin and
norfloxacin; effect reduced by rifamycins.
Anticoagulants: effect possibly enhanced by
coumarins; also possibly changes to INR.
Antifungals: concentration increased by fluconazole
and miconazole and possibly voriconazole.
Bosentan: increased risk of hepatoxicity - avoid.
Ciclosporin: may increase ciclosporin levels.
Lipid-regulating drugs: absorption reduced by
colesevelam; concentration possibly increased by
fluvastatin; possibly additive hypoglycaemic effect
with fibrates.
Sulfinpyrazone: enhanced effect of sulphonylureas. | | Metabolism | Glibenclamide is metabolised, almost completely, in the
liver, the principal metabolite being only very weakly
active.
About 50% of a dose is excreted in the urine and 50% via
the bile into the faeces. | | storage | Store at RT | | References | 1) Brogden et al. (1979), Glipizide: a review of its pharmacological properties and therapeutic use; Drugs, 18 329
2) Sheppard and Welsh (1992), Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents; J. Gen. Physiol., 100 573 |
| | Glibenclamide Preparation Products And Raw materials |
|



