|
| Product Name: | Tungsten trioxide | | Synonyms: | CI 77901;TUNGSTEN TRIOXIDE 99.999%;TUNGSTEN TRIOXIDE, 99.5%;Tungsten(VI)oxide(99.9994%-W)PURATREM;Tungsten(VI)oxide(min.99.8%-W);Tungsten(VI)oxide,sinteredlumps(99.99%-W)PURATREM;tungsten(vi) oxide, puratronic;TUNGSTENOXIDE,POWDER | | CAS: | 1314-35-8 | | MF: | O3W | | MW: | 231.84 | | EINECS: | 215-231-4 | | Product Categories: | metal oxide;Inorganics | | Mol File: | 1314-35-8.mol |  |
| | Tungsten trioxide Chemical Properties |
| Melting point | 1470-1475 °C | | Boiling point | 1837°C (estimate) | | density | 7.16 g/mL at 25 °C(lit.) | | storage temp. | Room Temperature | | form | powder | | color | Yellow to greenish-yellow | | Specific Gravity | 7.16 | | Water Solubility | insoluble | | Merck | 14,9817 | | Exposure limits | ACGIH: TWA 3 mg/m3
NIOSH: TWA 5 mg/m3; STEL 10 mg/m3 | | InChIKey | ZNOKGRXACCSDPY-UHFFFAOYSA-N | | CAS DataBase Reference | 1314-35-8(CAS DataBase Reference) | | NIST Chemistry Reference | Tungsten trioxide(1314-35-8) | | EPA Substance Registry System | Tungsten trioxide (1314-35-8) |
| Hazard Codes | Xn | | Risk Statements | 22-36/37/38 | | Safety Statements | 26-36-37/39 | | WGK Germany | - | | RTECS | YO7760000 | | F | 21 | | TSCA | Yes | | HS Code | 28259012 | | Hazardous Substances Data | 1314-35-8(Hazardous Substances Data) | | Toxicity | rat,LD50,oral,1059mg/kg (1059mg/kg),BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)BEHAVIORAL: EXCITEMENTBEHAVIORAL: MUSCLE WEAKNESS,Hygiene and Sanitation Vol. 31(7-9), Pg. 197, 1966. |
| | Tungsten trioxide Usage And Synthesis |
| Description | Tungsten trioxide is a chemical compound consisting of oxygen and
the transition metal tungsten. It occurs naturally but rarely in the
form of hydrates in some kinds of minerals. It has various
applications. In industry, it can be used for making x-ray screen
phosphors, fireproofing fabrics and gas sensors as well as a pigment
in ceramics and paints. It can also be used for the preparation of
electrochromic windows or smart windows. In addition, it can be used
in semiconductors. It, together with titanium dioxide, has the
potential to become an efficient photo-catalyst under visible light
irradiation. Recent study has shown that mesoporous tungsten
trioxide polyaniline nanocomposite can be used as an anode material
for high-performance lithium-ion batteries. | | Chemical Properties | Tungsten trioxide is a bright canary-yellow coloured powder which becomes dark-orange on heating, but regains its bright yellow colour on cooling. A very slight admixture of sodium salt imparts to the oxide a greenish tint which no amount of oxidation can remove (Roscoe). It also becomes greenish on exposure to light. Tungsten trioxide has been obtained in the crystalline state by Debray, by igniting a mixture of tungstate and carbonate of sodium in a current of hydrochloric acid, when the trioxide is obtained in olive-green rectangular prisms which sublime at a white heat. The crystalline trioxide has also been prepared by heating hydrated tungstic acid with borax in a porcelain furnace (Nordenskjold). The specific gravity of tungsten trioxide thus obtained is 6.34.

When heated in a current of hydrogen to 250° the trioxide is converted into the blue oxidc, 2WO3+WO2 and if the heat be raised to dull redness the brown oxide, WO2 is formed, whilst at a higher temperature the metal is obtained. Acted upon by reducing agents such as zinc and hydrochloric acid, stannous chloride, or organic matter, tungsten trioxide is trans-formed successively into the blue and brown oxides.
| | hermochromism | hermochromism is the property of substances to changecolour due to a change in temperature and WO3is an attractive example for this property. On cooling the oxide by liquid nitrogen down to -196°C, a sudden change occurs from yellow to white, which then alters to a bluish-white colour between -50 and -27°C. At room temperature it becomes pale lemon-yellow again. On furtherheating to 200 – 300°C, WO3becomes dark yellow, changingto a deep orange colour at 400 to 500°C .
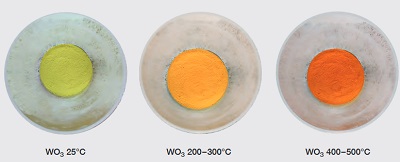
These reversible colour changes are linked to changes in the electronic properties of WO3 which alter due to changesof the internal symmetry of the WO3crystals (through alterations of the lattice arrangements).
| | Uses | Tungsten trioxide is a thermally stable and water insoluble tungsten compound. Tungsten trioxide andTungsten blue oxides are both used in the production of tungsten metal powders. The greatmajority of metallic tungsten is used to make cemented carbide parts, and theremainder is used in manufacturing components for lighting, electrical, electronic,heating, and welding applications; and in tool steels, alloys, catalysts, pigments, andhigh-temperature lubricants.
| | Preparation | Yellow tungsten trioxide is a finely divided, yellow, crystalline powder. It is produced by roasting ammonium paratungstate at closely controlled temperatures to drive off combined water and ammonia. | | References | [1]Momeni, Mohamad Mohsen, and Yousef Ghayeb. "Photochemical deposition of platinum on titanium dioxide–tungsten trioxide nanocomposites: an efficient photocatalyst under visible light irradiation." Journal of Materials Science: Materials in Electronics 27.2 (2016): 1062- 1069.
[2]Li, Bin, et al. "Mesoporous Tungsten Trioxide Polyaniline Nanocomposite as an Anode Material for High‐Performance Lithium‐ Ion Batteries."ChemNanoMat 2.4 (2016): 281-289.
[3]Hunge, Y. M., et al. "Photoelectrocatalytic degradation of methyl blue using sprayed WO3 thin films." Journal of Materials Science: Materials in Electronics 27.2 (2016): 1629-1635.
[4]Albalshi, Madleen Ahmad Mohammad. Electrochemical properties of Sol-gel WO3 films Co-doped with Ti and Zn. Diss. 2016.
| | Chemical Properties | Canary-yellow, heavy powder, darkorange
when heated and regains original color on
cooling. Insoluble in water, soluble
in caustic alkalies, soluble with difficulty in
acids. Noncombustible. | | Physical properties | Heavy yellow powder; turns dark orange on heating; reverts back to yellow on cooling; density 7.2 g/cm3; melts at 1,472°C; insoluble in water; slightly soluble in acids; soluble in caustic alkalies. | | Uses | manufacture of tungstates which are used for x-ray screens and for fireproofing fabrics. | | Uses | Tungsten oxide (WO3) is used to make tungsten alloys. Tungsten oxide is also used as fireproofing
for various surfaces and is used as a yellow pigment in ceramics. | | Uses | It is used in tungsten and tungstate manufacturing which are used as X-ray screens and for fire proofing fabrics. It is used as a ceramic pigment. Nanowires of Tungsten (VI) oxide are capable of absorbing a higher percentage of the sun radiation since it absorbs blue light. | | Preparation | Tungsten trioxide is obtained as an intermediate in recovery of tungsten from its minerals (See Tungsten). In commerical processes tungstic acid, H2WO4, obtained from the mineral scheelite, may either be decomposed at high temperatures to form trioxide or dissolved in ammonium hydroxide solution and evaporated to yield ammonium paratungstate (APT) crystals, 5 (NH4)2O?12WO3?11H2O. The APT crystals are then washed, dried, and calcined at elevated temperatures to form tungsten trioxide
Tungsten trioxide, in general, can be made by heating metallic tungsten, its carbides, its lower oxides, or tungstic acid in air. | | General Description | Tungsten(VI) oxide (WO3) is a semiconducting material with excellent electrochromic properties. It is a cost efficient material with a high optical modulation. It can be synthesized by a variety of methods which include chemical vapor deposition, electrodeposition, and other sol-gel based coating processes. | | Hazard | Toxic material. | | Flammability and Explosibility | Notclassified | | Safety Profile | Moderately toxic by
ingestion. Can react violently with ClF3, Li,
Cl2. See also TUNGSTEN COMPOUNDS. | | Structure and conformation | The crystal structure of tungsten trioxide is temperature dependent. It is tetragonal at temperatures above 740℃, orthorhombic from 330 to 740 °C, monoclinic from 17 to 330 °C, triclinic from -50 to 17 °C, and monoclinic again at temperatures below -50 °C.The most common structure of WO3 is monoclinic with space group P21/n.
|
| | Tungsten trioxide Preparation Products And Raw materials |
|




