| uses | diazo-transfer reagent for activated methylene groups. |
| Uses | Reactant or reagent for synthesis of:
- Positron emission tomography ligand for imaging metabotropic glutamate receptor type 1
- Stitching agent for introduction of aziridines into functionalized organic molecules
- Parenteral cephalosporins
Reagent for:
- Intermolecular metal-catalyzed carbenoid cyclopropanations
- Solid-phase synthesis of indolecarboxylates using palladium catalyzed reactions
- Solid-phase rhodium carbenoid N-H insertion reactions
|
| Uses | 4-Dodecylbenzensulfonyl Azide is useful for the synthesis of N-(Benzo[h]quinolin-10-yl)-4-dodecylbenzenesulfonamide. Also, it is used in the preparation of ruthenium nanoparticles protected by ruthenium-nitrene n bonds. |
| Preparation | prepared by addition of granular sodium azide (1.3 equiv) to a mixture of isomeric sulfonyl chlorides (from a commercial mixture of isomers of p-dodecylbenzenesulfonic acid) in acetone solution. |
| Reactions | Reagent for Diazo-function Transfer.
The title reagent has been called a safer diazo-transfer reagent, and is a mixture of 12 or more isomeric p-dodecylbenzenesulfonyl azides, ranging by HPLC from 24% to 1% in area and giving essentially a single spot by TLC.
The title reagent has been shown to be very useful for the synthesis of various crystalline diazocarbonyl compounds such as diazobarbituric and diazoisopropylidenemalonic acids, derivatives and homologs of diazoacetoacetic acid, and 9-diazoanthrone (eq 1).
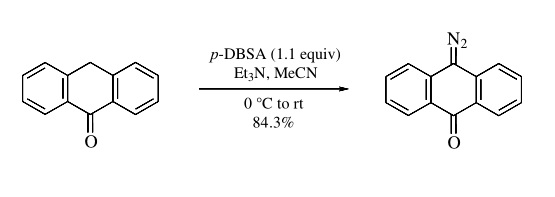
Formation of noncrystalline byproduct dodecylsulfonamides facilitates the workup procedure and isolation of the target crys- talline diazo compounds from the reaction mixture. |
| storage | Dodecylbenzenesulfonyl azide is the safest of the usual are nesulfonyl azides (tosyl azide, etc.) employed in diazo-transfer processes;appropriate care should be taken,as with all azides. Use in a fume hood. |
| Purification Methods | elution through short column containing silica gel G with methylene chloride-hexane (1:4) as eluant. |