|
| | Teneligliptin Hydrobromide Basic information |
| Product Name: | Teneligliptin Hydrobromide | | Synonyms: | [(2S,4S)-4-[4-(5-methyl-2-phenylpyrazol-3-yl)piperazin-1-yl]pyrrolidin-2-yl]-(1,3-thiazolidin-3-yl)methanone,pentahydrobromide;API(Teneligliptin hydro bromide hydrate);3-(((2s,4s)-4-(4-(3-Methyl-1-phenyl-1h-pyrazol-5-yl)-1-piperazinyl)-2-pyrrolidinyl)carbonyl)-thiazolidine hydrobromide (2:5) X H2O;Teneligliptin (hydrobroMide);Teneligliptin HydrobroMide Hydrate;3-[[(2S,4S)-4-[4-(3-Methyl-1-phenyl-1H-pyrazol-5-yl)-1-piperazinyl]-2-pyrrolidinyl]carbonyl]-thiazolidine hydrobromide (2:5);Teneligliptin HydrobroMide (2:5);Teneligliptin HBr | | CAS: | 906093-29-6 | | MF: | C22H31BrN6OS | | MW: | 507.5 | | EINECS: | | | Product Categories: | API | | Mol File: | 906093-29-6.mol |  |
| | Teneligliptin Hydrobromide Chemical Properties |
| Melting point | >211°C (dec.) | | storage temp. | Refrigerator | | solubility | Methanol (Slightly) | | form | Solid | | color | White to Light Beige |
| | Teneligliptin Hydrobromide Usage And Synthesis |
| Uses | Teneligliptin Hydrobromide (2:5) is a dipeptidyl peptidase-4 (DPP-4) inhibitor that is used to treat type 2 diabetes. It is eliminated via excretion, and has a half-life of 24.2 hours in the human body. | | Biological Activity | teneligliptin hydrobromide is a novel, potent, and long-lasting dipeptidyl peptidase-4 (dpp-4) inhibitor with antioxidant properties; competitively inhibited human plasma, rat plasma, and human recombinant dpp-4 in vitro, with ic50 values of about 1 nm[1].dpp4 is also known as adenosine deaminase complexing protein 2 or cd26. dpp4 is an antigenic enzyme expressed on the membrane of most cell types and is associated with immune regulation, signal transduction and apoptosis [2]. | | Clinical Use | Teneligliptin is a DPP-4 inhibitor which was approved in Japan in 2012 for the treatment of type II
diabetes. It was discovered and developed by Mitsubishi Tanabe Pharma under the trade name
Tenelia®. Similar to other marketed DPP-4 inhibitors, teneligliptin was well tolerated in all studies and
QD dosing produced a long-lasting inhibitory action against DPP-4 and an increase in active GLP-1
levels, with very low rates of renal excretion. | | Synthesis | The only reported synthesis of teneligliptin is described in the scheme below. Reaction of commercially
available N-Boc-piperazine (158) with diketene (159) in DMF at room temperature gave acetoacetamide
160 in 86% yield, and this material was immediately condensed with phenylhydrazine in
methanesulfonic acid followed by a cyclodehydration with phosphorus oxychloride to give pyrazole 161
in 12% yield. The t-butyl carbamate was then removed with TFA in dichloromethane to give amine 162
in 88% yield. This amine was then subjected to butyrolactam 165 (which was prepared from N-Boctrans-
4-hydroxy-L-proline (163) coupled with thiazolidine (164) under conventional amide-forming
conditions using EDC) in the presence of sodium triacetoxy borohydride (STAB-H) in acetic acid.
This reductive amination reaction afforded the cis-aminopyrrolidine 166 exclusively in 50% yield.
Removal of the t-butyl carbamate group with TFA afforded the teneligliptin free amine in 93% yield,
and this freebase was then subsequently treated with 48% hydrobromic acid in refluxing ethanol to give
teleligliptin hydrobromide hydrate (XXV) in 90% yield. 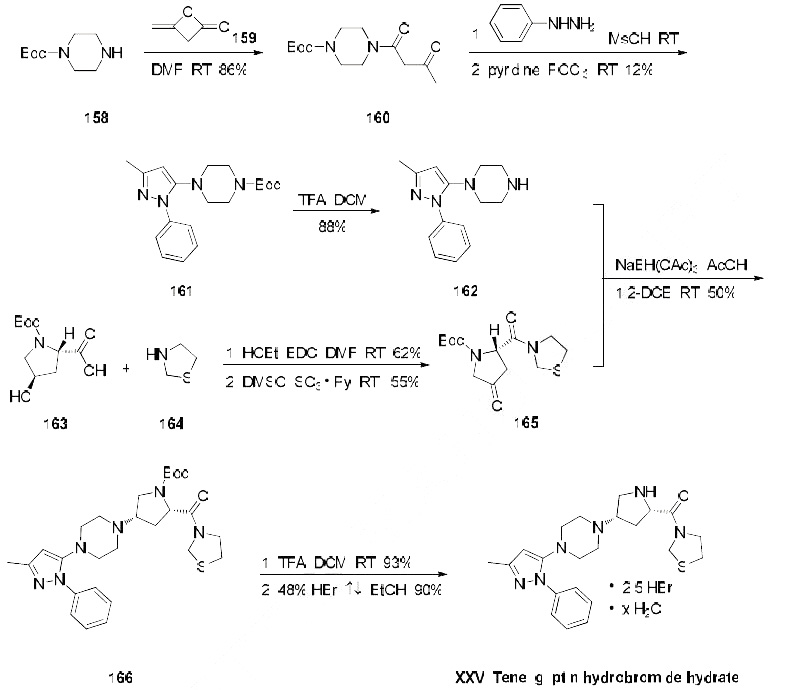
| | in vitro | in human umbilical vein endothelial cells, teneligliptin promoted the antioxidant response, reduced ros levels and induced nrf2-target genes messenger ribonucleic acid expression. teneligliptin improved proliferation rates in huvecs exposed to high glucose, regulating the expression of cell-cycle inhibitors markers (p21, p53 and p27), and reducing proapoptotic genes (bax and casp3), while promoteed bcl2 expression. teneligliptin ameliorated high glucose-induced endoplasmic reticulum stress. teneligliptin exihibited antioxidant properties and overcome the metabolic memory effect induced by chronic exposure to high glucose in huvecs[3]. | | in vivo | in teneligliptin-treated mice, serum alanine aminotransferase and intrahepatic triglyceride levels were significantly decreased (p < 0.05). teneligliptin also significantly downregulated hepatic mrna levels of the genes involved in de novo lipogenesis (p < 0.05). moreover, the hepatic expression levels of phosphorylated amp-activated protein kinase (ampk) protein were upregulated by teneligliptin[4]. orally administration of teneligliptin at a dosage of 20 mg once daily in adults in the ovariectomized (ovx) mice maintained on a high-fat diet, teneligliptin effectively ameliorated the characteristics of metabolic abnormalities associated with postmenopausal obesity. teneligliptin ameliorated the decreased energy consumption in the dark and light phases, reduced locomotor activity in the dark phase, and lowered core body temperature in ovx-hf[5]. | | references | [1] kishimoto m. teneligliptin: a dpp-4 inhibitor for the treatment of type 2 diabetes[j]. diabetes, metabolic syndrome and obesity: targets and therapy, 2013, 6: 187.
[2] kameoka j, tanaka t, nojima y, et al. direct association of adenosine deaminase with a t cell activation antigen, cd26[j]. science, 1993, 261(5120): 466-469.
[3] pujadas g, de nigris v, prattichizzo f, et al. the dipeptidyl peptidase-4 (dpp-4) inhibitor teneligliptin functions as antioxidant on human endothelial cells exposed to chronic hyperglycemia and metabolic high-glucose memory[j]. endocrine, 2016: 1-12.
[4] ideta t, shirakami y, miyazaki t, et al. the dipeptidyl peptidase-4 inhibitor teneligliptin attenuates hepatic lipogenesis via ampk activation in non-alcoholic fatty liver disease model mice[j]. international journal of molecular sciences, 2015, 16(12): 29207-29218.
[5] sameshima a, wada t, ito t, et al. teneligliptin improves metabolic abnormalities in a mouse model of postmenopausal obesity[j]. journal of endocrinology, 2015, 227(1): 25-36. |
| | Teneligliptin Hydrobromide Preparation Products And Raw materials |
|