| Description | Amsacrine is a cytostatic reported to be active against adult lymphoblastic leukemia
which has failed primary treatment or become resistant. Its clinical use, however, is
associated with significant neuro-, gastro- and hepatotoxicity. |
| Originator | Auckland Cancer Chemotherapy Lab (New Zealand) |
| Uses | Amsacrine (Amsidyl) is used as an Investigational drug. |
| Uses | Amsacrine is a drug undergoing intensive trials for severe leukemia and lymphoma. It is a
cytotoxic drug that binds with DNA with expressed specificity to the adenosine–tyrosine
pair, thus inhibiting DNA synthesis. It has been suggested to be used for severe leukemia.
A synonym of this drug is amsidyl. |
| Uses | Antineoplastic. |
| Definition | ChEBI: A sulfonamide that is N-phenylmethanesulfonamide substituted by a methoxy group at position 3 and an acridin-9-ylamino group at position 4. It exhibits antineoplastic activity. |
| Brand name | Amsidyl (Parke-Davis);Amsakrin. |
| Synthesis | Amsacrine, 4-(9-acridinylamino)-3-methoxyphenyl-N-methansulfonamide
(30.6.11), is made by sulfonating 4-nitro-m-anisidine with methanesulfonyl chloride,
which forms a sulfonyl amide 30.6.9, and the nitro group is reduced to an amino group by
hydrogen, forming 4-amino-3-methoxyphenyl-N-methansulfonamide (30.6.10). Reacting
this with 9-chloroacridine gives amsacrine (30.6.11). 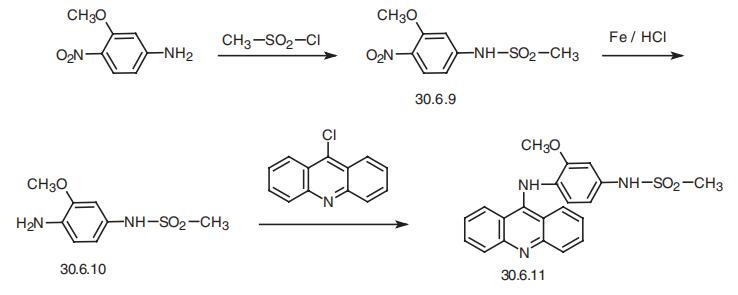
|
| references | [1] nelson em, tewey km, liu lf. mechanism of antitumor drug action: poisoning of mammalian dna topoisomerase ii on dna by 4'-(9-acridinylamino)-methanesulfon-m-anisidide. proc natl acad sci u s a. 1984 mar;81(5):1361-5. |



