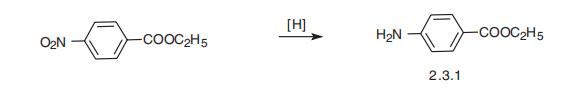|
| | Benzocaine Chemical Properties |
| Melting point | 88-90 °C | | Boiling point | 172 °C (12.7517 mmHg) | | density | 1.17 | | refractive index | 1.5600 (estimate) | | Fp | 172°C/13mm | | storage temp. | 2-8°C | | solubility | alcohol: soluble1 gm in 5 ml | | pka | 2.5(at 25℃) | | form | Crystalline Powder | | color | White | | Odor | odorless | | Water Solubility | Soluble in ethanol, chloroform, ethyl ether and dilute acids. Sparingly soluble in water | | Merck | 14,1086 | | BRN | 638434 | | Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. | | InChIKey | BLFLLBZGZJTVJG-UHFFFAOYSA-N | | LogP | 1.860 | | CAS DataBase Reference | 94-09-7(CAS DataBase Reference) | | NIST Chemistry Reference | Benzoic acid, 4-amino-, ethyl ester(94-09-7) | | EPA Substance Registry System | Benzocaine (94-09-7) |
| | Benzocaine Usage And Synthesis |
| Indications and Usage | Benzocaine is a colorless trapezial crystal. Its melting point is 92℃ (88-90℃), boiling point is 183-184°C (1.87kPa). 1g of this drug is soluble in about 2500ml water, 5ml ethanol, 2ml chloroform, 4ml ether or 30-50ml almond oil and olive oil, and it is also soluble in dilute acid. It is stable in air, odorless, and slightly bitter.
Benzocaine is a lipid-soluble surface anesthetic, and it weaker than other local anesthetics such as lidocaine and tetracaine, so it will not cause any discomfort due to its anesthetizing effects when acting on mucosa. Benzocaine is a type of drug with relatively strong lipid-solubility and will bind with mucosa and the fatty layer of skin, but it will not easily penetrate into the body and cause poisoning. Benzocaine can be used as a precursor for Ossur imitation, orthocaine, and procaine. It is also used as a local anesthetic and can stop pain and itching. It is mainly used in pain and itch prevention on wounds, ulcer surfaces, mucous membrane surfaces and hemorrhoids. Its paste form can also lubricate and stop pain for the nasopharynx and endoscope. Benzocaine’s aural solution is used to alleviate acute congestion, concentrated otitis externa, and the pain and itching of swimming otitis. Benzocaine is also effective for toothaches, sore throat, oral ulcers, all kinds of hemorrhoids, anal fissures, and vulvar itching. Benzocaine can be used as a male sex organ numbing agent to slow ejaculation. It can also be used as a numbing lubricant for the pharynx and endoscope, and it can be used as a UV absorbing agent for cosmetics.
| | Uses |
- Benzocaine is a local anaesthetic of the ester type with a poor solubility in water. The drug benzocaine is normally used as a topical pain reliever or as a common ingredient in cough drops. Benzocaine is used in multiple forms including lotion, gel, liquid, lozenges, and sprays. When Benzocaine is applied in any form it temporarily numbs or blocks the nerve endings, which leads to a decreases in the amount of pain. It is used in cattle, sheep, swine and horses for local and prolonged low epidural anaesthesia. Standard therapeutic doses of benzocaine ranged from 150 to 750 mg per animal. Benzocaine is also currently used as surface anaesthetic as ointments (0.5% benzocaine) for wounds and ulcerated surfaces in horses, cattle and sheep applied twice a day until healing.
- A commonly used topical pain reliever. Main active ingredient in anesthetic ointments.
- Anesthetic;Na+ channel blocker
- Benzocaine acts on the central nervous system, cardiovascular system, neuromuscular junctions and ganglion synapse. Its mechanism of action is to prevent the generation and conduction of the nerve impulse. Local anaesthetics block conduction by decreasing or preventing the large transient increase in the permeability of excitable membranes to Na+ that is produced by a slight depolarisation. This action of local anaesthetics is due to their direct interaction with voltage-sensitive Na+ channels. Benzocaine is mainly hydroxylated in the metabolite para-aminobenzoic acid (PABA) that inhibits the action of sulphonamides.
- Benzocaine is distributed in the body, crosses the placenta and is metabolised in the liver and in the plasma by non-specific cholinesterases. Benzocaine and its main metabolite (para-aminobenzoic acid) are excreted into urine.
- Benzocaine appears as a moderately toxic compound after single administration. The acute intraperitoneal LD50 value was 216 mg/kg bw in mice. Benzocaine may induce methemoglobinemia in several species such as sheep, cats and dogs.
| | synthesis | Benzocaine is produced by reduction of ethyl 4-nitrobenzoate with iron or by the acid-catalyzed esterification of 4-aminobenzoic acid with ethanol:

In a 5-mL round bottom flask, add 120 mg of 4-aminobenzoic acid (often called PABA for paminobenzoic acid), 1.5 mL of absolute ethanol (absolute ethanol is completely free of water and can be found on the hooded shevles), and 3 boiling chips. Heat this mixture on a sand bath until all the solid dissolves. Cool in ice and then add 0.25 mL of concentrated sulfuric acid dropwise. (One drop at a time.) A large amount of precipitate will form when the sulfuric acid is added, but this will dissolve during the reflux that follows. Attach an air condenser from the microscale kit, and reflux gently for 60- 75 min. Check periodically to be sure that the mixture is refluxing gently, and that the ring of condensation of solvent lies somewhere along the inner surface of the air condenser; loss of solvent will cause overheating and a significant decrease in yield. | | Description | Benzocaine (ethyl p-aminobenzoate) is used topically by itself or in combination with menthol or
phenol in nonprescription dosage forms such as gels, creams, ointments, lotions, aerosols, and
lozenges to relieve pain or irritation caused by such conditions as sunburn, insect bites,
toothache, teething, cold sores or canker sores in or around the mouth, and fever blisters.
Benzocaine is a lipophilic local anesthetic agent with a short duration of action.
Like most amino ester-type local anesthetics, it is easily hydrolyzed by plasma cholinesterase.
Because of its low pKa, however, it is un-ionized under most physiological conditions and, therefore, can only bind
to the lipid side of the local anesthetic receptor. It also can easily cross membranes
into systemic circulation to cause systemic toxicities. Furthermore, being a PABA derivative, it
has similar allergenic properties to procaine and is contraindicated with sulfonamide antibacterial
agents. | | Chemical Properties | Benzocaine is a white odourless needle-like crystal, slightly soluble in water and easily soluble in organic solvents. For example: ethanol, chloroform, ether, soluble in almond oil, olive oil. | | Uses | Benzocaine is used as an anesthetic (Local and topical) that in products such as burn and sunburn remedies, hemorrhoidal creams, suppositories, creams for treatment of poison ivy, oral and gingival products, sore-throat sprays/lozenges, astringents, appetite suppressants, callus and wart remedies, athlete's-foot remedies, toothache- and denture-irritation products. | | Definition | ChEBI: Benzocaine is a benzoate ester having 4-aminobenzoic acid as the acid component and ethanol as the alcohol component. A surface anaesthetic, it is used to suppress the gag reflex, and as a lubricant and topical anaesthetic on the larynx, mouth, nasal cavity, respiratory tract, oesophagus, rectum, urinary tract, and vagina. It has a role as a topical anaesthetic, an antipruritic drug, an allergen and a sensitiser. It is a benzoate ester and a substituted aniline. | | Brand name | Americaine (Fisons); Baby
Anbesol (Whitehall-Robins). | | Biological Functions | Benzocaine is a PABA derivative used primarily for
topical application to skin and mucous membranes. Its
low aqueous solubility allows it to stay at the site of application
for long periods. Its minimal rate of absorption
after topical administration is associated with a low incidence
of systemic toxicity. Benzocaine is contraindicated
in patients with known sensitivity to ester-linked
anesthetics or PABA-containing compounds. | | Synthesis Reference(s) | Journal of the American Chemical Society, 71, p. 4154, 1949 DOI: 10.1021/ja01180a513
Chemical and Pharmaceutical Bulletin, 29, p. 1443, 1981 DOI: 10.1248/cpb.29.1443 | | General Description | Benzocaine is a unique local anesthetic because it does notcontain a tertiary amine. The pKa of the aromatic amine is 3.5ensuring that benzocaine is uncharged at physiological pH.Because it is uncharged, it is not water soluble but is ideal fortopical applications. The onset of action is within 30 secondsand the duration of drug action is 10 to 15 minutes. | | Biochem/physiol Actions | Benzocaine is the ethyl ester of p-aminobenzoic acid (PABA). Benzocaine acts to inhibit the voltage-dependent sodium channels (VDSCs) on the nerve membrane, stopping the propagation of the action potential. | | Clinical Use | Benzocaine is used for endoscopy, bronchoscopy, and topicalanesthesia. Benzocaine is available as a 20% solution topicalspray, in a 1% gel for mucous membrane application, and a14% glycerin suspension for topical use in the outer ear.Toxicity to benzocaine can occur when the topical doseexceeds 200 to 300 mg resulting in methemoglobinemia.Infants and children are more susceptible to this and methemoglobinemiahas been reported after benzocaine lubricationof endotracheal tubes and after topical administration to treata painful diaper rash. | | Safety Profile | Poison by ingestion and
intraperitoneal routes. Human systemic
effects by rectal route:
methemoglobinemia/carboxyhemoglobinem
ia in infants. A skin irritant and a mild
sensitizer. Local contact may cause contact
dermatitis. Used as a topical anesthetic and
as a sun-screening agent. When heated to
decomposition it emits highly toxic fumes of
NOx. See also ETHYL ALCOHOL and
ESTERS | | Synthesis | Benzocaine is the ethyl ester of 4-aminobenzoic acid (2.3.1). The classic,
optimal way of benzocaine synthesis is the reduction of the nitro group of the ethyl ester
of 4-nitrobenzoic acid to benzocaine by hydrogen, which generates directly in the reaction
medium by the reaction of iron filings with dilute acids [24¨C26]. 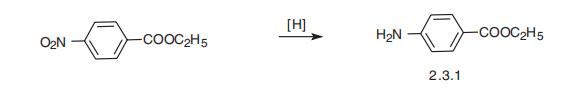
| | Purification Methods | Crystallise Benzocaine from EtOH/H2O or EtOH (m 93-94o), and dry it in air. [Beilstein 14 H 422, 14 IV 1129.] | | Structure and conformation | Ethyl aminobenzoate, a para-aminobenzoate derivative (PABA) |
| | Benzocaine Preparation Products And Raw materials |
|