|
| | Polybutene Basic information |
| Product Name: | Polybutene | | Synonyms: | 1-butene polymer (polybutene);Polybutene;PB2400;poly(ethylethylene);Poly-alpha-butylene;POLY(1-BUTENE);POLY(1-BUTENE), ISOTACTIC;POLY(1-BUTENE) ISOTACTIC AVERAGE MW C& | | CAS: | 9003-28-5 | | MF: | C4H8 | | MW: | 56.10632 | | EINECS: | | | Product Categories: | Polymers | | Mol File: | 9003-28-5.mol |  |
| | Polybutene Chemical Properties |
| | Polybutene Usage And Synthesis |
| Chemical Properties | Polybutene is generally water-white, reaching less than 50 on APHA color scale. (typically less than 10) The product remains very stable after being prolonged exposure to UV light. Polybutene is more stable than conventional mineral oil with same APHA color.
Polybutene is a non-polar hydrocarbon polymer hence it has high solubility in aliphatic, aromatic and chlorinated solvents. It is compatible with a wide variety of organic materials, for instance, synthetic hydrocarboon polymers including wax, rosin, asphalt and gum.
Polybutene is safe to use, because it is non-toxic. Polybutene is allowed by the FDA for a wide variety of applications as components of articles.
| | Chemical Properties |
Polybutene (polybutylene) (PB) is manufactured by stereospecific Ziegler Natta polymerization of l-butene:
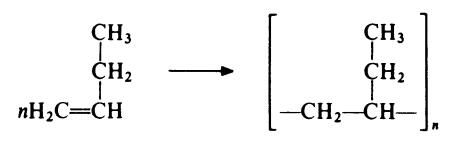 Commercial products are predominantly isotactic (98-99.5%) and of high molecular weight (Mw=2.3 x 105-7.5 x 105). An unusual feature of the polymer is that it can exist in several distinct crystalline forms. Crystallization from the melt yields the metastable Form II, which changes to the stable Form lover a period of 5 to 7 days at room temperature. Other crystalline forms maybe obtained from solution. Form I has a crystalline melting point of 135°C and a density of 0.95 g/cm3 . Form II has a crystalline melting point of 124°C and a density of 0.89 g/cm3.
Polybutene has properties to be expected from a crystalline polyolefin. It has a melting point and stiffness between those of high density and low density polyethylene. Its thermal stability lies between that of polyethylene and polypropylene. The polymer exhibits resistance to oxidizing and chemical environments broadly similar to that shown by polypropylene; like polypropylene, polybutene is immune from environmental stress cracking. An outstanding property of poly butene is its high creep resistance, due probably to the prevention of slippage of the polymer chains by the large number of ethyl side chains and the high molecular weight of commercial polymers. The main commercial application of polybutehe is in extruded pipe, which has good resistance to rupture under pressure. Such pipe finds use in hot and cold water plumbing and for the transport of abrasive or corrosive materials.
Commercial products are predominantly isotactic (98-99.5%) and of high molecular weight (Mw=2.3 x 105-7.5 x 105). An unusual feature of the polymer is that it can exist in several distinct crystalline forms. Crystallization from the melt yields the metastable Form II, which changes to the stable Form lover a period of 5 to 7 days at room temperature. Other crystalline forms maybe obtained from solution. Form I has a crystalline melting point of 135°C and a density of 0.95 g/cm3 . Form II has a crystalline melting point of 124°C and a density of 0.89 g/cm3.
Polybutene has properties to be expected from a crystalline polyolefin. It has a melting point and stiffness between those of high density and low density polyethylene. Its thermal stability lies between that of polyethylene and polypropylene. The polymer exhibits resistance to oxidizing and chemical environments broadly similar to that shown by polypropylene; like polypropylene, polybutene is immune from environmental stress cracking. An outstanding property of poly butene is its high creep resistance, due probably to the prevention of slippage of the polymer chains by the large number of ethyl side chains and the high molecular weight of commercial polymers. The main commercial application of polybutehe is in extruded pipe, which has good resistance to rupture under pressure. Such pipe finds use in hot and cold water plumbing and for the transport of abrasive or corrosive materials.
| | Physical properties | Polybutenes are light colored, nondrying, sticky, viscous liquids. They are stable when exposed to light, insoluble in water, and soluble in hydrocarbon and chlorinated hydrocarbon solvents. Polybutene-1 has a density of 0.92g/cm3 and a melting range of 124°C-130°C.
Polybutene is available in a variety of grades. The differences depend on viscosity, which increases directly in proportion to increasing molecular weight.
Polybutene films have a high resistance to stress cracking, and low stress deformation. | | Uses | polybutene is a binder and viscosity-increasing agent used more in makeup than skin care preparations. Polybutene is a polymer of one or more butylenes obtained from petroleum oils.
| | Application | Fuel and lubricant additives – Fuel dispersants, lubricant dispersants
Adhesives
Caulk and sealants
Gum base
Wrap film
Lubricants – Two-stroke engine oil, lubricant for rolling wire drawing, compressor oil and viscosity index improvers (VIIs)
Electrical insulation
Others – Asphalt modifier, resin plasticiser, rubber modifier and dispersing agents | | Definition | ChEBI: But-1-ene is a butene with unsaturation at position 1. | | Preparation | Passman describes an industrial preparation of Polybutene in which a solution of 1-butene is dried and fed into a reaction chamber. A Zeigler-Natta reagent (TiCI3, and diethyl aluminum chloride) is added to catalyze the polymerization process. The molecular weight of the end product is limited by the reaction temperature used in the chamber. | | Health Hazard | Recommended Personal Protective Equipment: Goggles or face shield; Symptoms Following Exposure: Low toxicity. Vapor may act as a simple asphyxiant in high concentrations; General Treatment for Exposure: INHALATION: remove victim from exposure; Toxicity by Inhalation (Threshold Limit Value): Data not available; Short-Term Inhalation Limits: Data not available; Toxicity by Ingestion: Grade 0, LD50 > 15 g/kg; Late Toxicity: None; Vapor (Gas) Irritant Characteristics: Vapors are nonirritating to the eyes and throat; Liquid or Solid Irritant Characteristics: No appreciable hazard. Practically harmless to the skin; Odor Threshold: Data not available. | | Chemical Reactivity | Polybutenes undergo combustion, pyrolysis, and autoxidation; the latter two can occur during analytical treatment. Their low polarity, low degree of unsaturation, and their closely packed, branched-chain molecular structure make Polybutenes resistant to chemical reaction. | | Toxicity evaluation | According to CTFA (2006b), laboratory tests with approved surrogate systems/animals revealed that skin contact testing showed only slight irritation (primary dermal irritation score [PDIS] of 1.5/8.0). There were no observed sensitivity reactions. Also, acute dermal irritation testing indicated that polybutenes are practically nontoxic because the LDso is greater than 10, 250 mg/kg. Lastly, polybutenes are relatively nontoxic when tested in an acute oral test (LDso > 34,600 mg/kg, rat).
In the Cosmetic Ingredient Review (CIR) safety assessment of the chemically related ingredient, Polybutene (Elder 1982), a 2-year chronic oral toxicity study of Polybutene H-IOO (75% concentrate) in Charles River albino rats was presented. The animals were separated into four groups of 60 (30 males and 30 females per group). The animals were given 0 (control), 800 (0.08%), 4000 (0.40%), or 20,000 (2.0%) ppm Polybutene blended into their regular diets. The rats were monitored for their body weights, mortality and reactions, tumor incidence, and hematologic, urologic, and pathologic changes. After 12 months of testing, five animals from each group were killed for evaluation. No gross or microscopic pathological changes could be COrrelated with Polybutene ingestion. No significant differences were found after 24 months of feeding in the body weights or weight of food consumption, hematological results, urology, or tumor formation between the animals fed Polybutene and those that were not. |
| | Polybutene Preparation Products And Raw materials |
|