|
| | Triphenylsilyl chloride Chemical Properties |
| Melting point | 91-94 °C(lit.) | | Boiling point | 378 °C | | density | 1.14 | | Fp | >200°C | | storage temp. | Refrigerator | | solubility | acetone: 0.1 g/mL, clear | | form | crystal | | Specific Gravity | 1.16 | | color | white | | Water Solubility | Reacts with water. | | Sensitive | Moisture Sensitive | | Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents | | BRN | 1820487 | | InChIKey | MNKYQPOFRKPUAE-UHFFFAOYSA-N | | CAS DataBase Reference | 76-86-8(CAS DataBase Reference) | | NIST Chemistry Reference | Triphenylchlorosilane(76-86-8) | | EPA Substance Registry System | Silane, chlorotriphenyl- (76-86-8) |
| Hazard Codes | C | | Risk Statements | 34-37 | | Safety Statements | 26-36/37/39-45-24/25 | | RIDADR | UN 3261 8/PG 2 | | WGK Germany | 1 | | RTECS | VV2720000 | | F | 10-21 | | TSCA | Yes | | HazardClass | 8 | | PackingGroup | II | | HS Code | 29310095 |
| | Triphenylsilyl chloride Usage And Synthesis |
| ?Acute toxicity | IV- mouse LD50: 56 mg/kg | | Description | Triphenylsilyl chloride is a white to off-white crystal or powder with acrid ordor of hydrogen chloride, used for synthesis of pharmaceutical intermediates. | | Physical properties | mp 92–94°C; bp 240–243°C/35 mmHg. | | Uses | A wide variety of Grignard reagents and organolithium complexes
participate in reactions with Ph3SiCl to afford organosilanes.
Thus the reaction of allylmagnesium chloride with Ph3SiCl gives the allylsilane in high yield (eq 3).However, when hydrosilyl
Grignard reagents are employed, reduction to the silane
occurs (eq 4).Azaallyl anion6 and diazolithium salts can each
be silylated to give the anticipated silane adducts (eqs 5 and 6).
Similarly, arylsilanes8 and alkynylsilanes are produced upon
silylation of the appropriate metalated13 carbanions with Ph3SiCl
(eqs 7–11).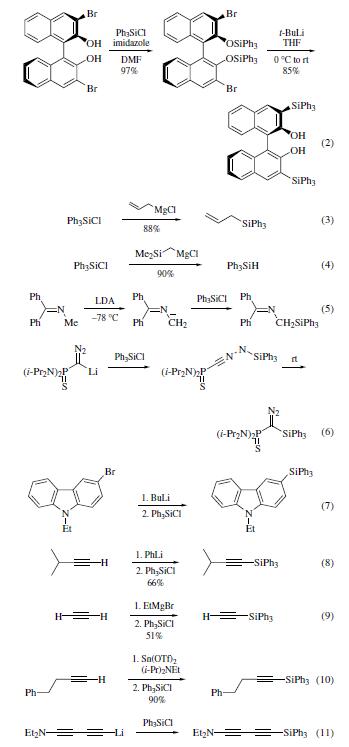 | | Uses | Chlorotriphenylsilane is used as a silylating agent. It is also used as a pharmaceutical intermediate. | | Purification Methods | Likely impurities are tetraphenylsilane, small amounts of hexaphenyldisiloxane and traces of triphenylsilanol. Purify it by distillation at 2mm, then crystallise it from EtOH-free CHCl3, and from pet ether (b 30-60o) or hexane by cooling in a Dry-ice/acetone bath. [Allen & Modena J Chem Soc 3671 1957, Curran et al. J Am Chem Soc 72 4471 1950, Speier & Zimmerman J Am Chem Soc 77 6395 1955, Thomas & Rochow J Am Chem Soc 79 1843 1957, Beilstein 16 IV 1484.] |
| | Triphenylsilyl chloride Preparation Products And Raw materials |
|



