|
| | 4-Methyl-2-hexanamine hydrochloride Basic information | | Uses |
| | 4-Methyl-2-hexanamine hydrochloride Chemical Properties |
| Melting point | 128.0 to 132.0 °C | | Fp | 9℃ | | storage temp. | room temp | | solubility | H2O: soluble5mg/mL (clear solution) | | form | powder | | color | white to beige | | InChI | InChI=1S/C7H17N.ClH/c1-4-6(2)5-7(3)8;/h6-7H,4-5,8H2,1-3H3;1H | | InChIKey | ZKKBPHUAHARETG-UHFFFAOYSA-N | | SMILES | C(C)(CC)CC(N)C.Cl | | CAS DataBase Reference | 13803-74-2(CAS DataBase Reference) |
| | 4-Methyl-2-hexanamine hydrochloride Usage And Synthesis |
| Uses | 1,3-Dimethylpentylamine is useful in composition in treating hypertrophied and hyperplastic gums which showed a significant reduction in swelling. | | Description | 1,3-Dimethylpentylamine is a pseudo-adrenergic drug introduced by Lilly
Company in the United States in the mid-1940s under the trade name
Forthane. 1,3-dimethylpentylamine and its hydrochloride have obvious
curative effects in the treatment of nasal congestion and gingival
hyperplasia, and are much less stimulating to the mind than
pseudoephedrine and amphetamine, and their unique pharmacological
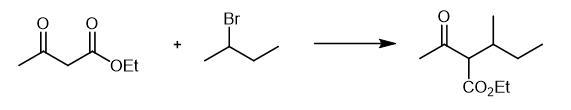
advantages cause attached great importance to the experts. | | Chemical Properties | White Solid | | Uses | 1,3-Dimethylpentylamine is useful in composition in treating hypertrophied and hyperplastic gums which showed a significant reduction in swelling. | | Uses | Methylhexanamine (hydrochloride) is a simple aliphatic amine that was once marketed as a nasal decongestant (Forthane) but is now sold as a bodybuilding supplement (Floradrene, Geranamine). It is also sold as a mild stimulant in the form of party pills and has been reported to cause cerebral hemorrhage in a case report. While little is known about its mode of action, methylhexanamine interferes in immunoassays for amphetamines, presumably because of the common ethylamine structure. This compound is intended for forensic or research purposes.[Cayman Chemical] | | Biochem/physiol Actions | Methylhexanamine is a naturally substance isolated from Pelargonium graveolen. It is a component of geranium oil. Methylhexanamine is a sympathomimentic that increase levels of norepinephrine in the synaptic cleft. | | Synthesis | At
room temperature, 800 mL absolute ethanol was added into a 2 L three
neck flask, stirred, and 35.38 g (1.538 mol) of metal sodium was added
in batches to obtain a colorless transparent liquid. Cool slightly, add
200g (1.537 rnol) of ethyl acetoacetate, continue heating slowly, and
add 232g (1.693 mol) of 2-bromobutane drop under weak reflux. Continue
heating and react for 8 h at 80~84 ??. Stop heating, cool naturally to
room temperature, leave it still, filter by suction, and concentrate the
yellow green filtrate under reduced pressure to obtain 241.4 g of 1,3-Dimethylpentanamine Hydrochloride with light
yellow viscous solid in 84.3% yield.
 |
| | 4-Methyl-2-hexanamine hydrochloride Preparation Products And Raw materials |
|