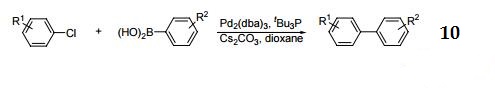|
| Product Name: | Tris(dibenzylideneacetone)dipalladium | | Synonyms: | Bis(dibenzylideneacetone)palladium(0)~Pd_2(dba)_3;Tris(dibenzylidenaceTone) dipalladium (O);Tris(dibezylideneacetone)dipalladium;Palladium, tris.mu.-(1,2-.eta.:4,5-.eta.)-(1E,4E)-1,5-diphenyl-1,4-pentadien-3-onedi-;Tris(dibenzylidenaceton)dipalladium;TRIS(BIBENZYLIDENEACETONE) DIPALLADIUM;Tris(dibenzylideneacetone)dipalladium(0), Pd 21.5% min;Tris(dibenzylideneacetone)dipalladium(0), Pd content 22-24% | | CAS: | 51364-51-3 | | MF: | C51H42O3Pd2 | | MW: | 915.71738 | | EINECS: | 610-654-4 | | Product Categories: | chemical reaction,pharm,electronic,materials;organometallic complexes;Catalysis and Inorganic Chemistry;Homogeneous Pd Catalysts;Catalysts-Ligands;Pd;Catalysts for Organic Synthesis;Classes of Metal Compounds;Homogeneous Catalysts;Metal Complexes;Pd (Palladium) Compounds;Synthetic Organic Chemistry;Transition Metal Compounds;Palladium | | Mol File: | 51364-51-3.mol |  |
| | Tris(dibenzylideneacetone)dipalladium Chemical Properties |
| Melting point | 152-155°C | | storage temp. | 2-8°C | | solubility | Soluble in chlorinated solvents, benzene and THF. | | form | Fine Crystalline Powder | | color | Purple to black | | Water Solubility | insoluble | | Sensitive | Air & Moisture Sensitive | | InChIKey | IBXMKLPFLZYRQZ-VCHVFRDLSA-N | | CAS DataBase Reference | 51364-51-3(CAS DataBase Reference) |
| | Tris(dibenzylideneacetone)dipalladium Usage And Synthesis |
| Description | Tris(dibenzylideneacetone) dipalladium (Tris DBA) is used as catalyst for a wide variety of Pd catalyzed reactions including Suzuki coupling, Heck coupling, Negishi coupling, Carroll reaarangement, Trost asymmetric allylic alkylation, Buchwald-Hartwig amination of acryl halides, fluorination of allylic chlorides, arylation of ketones, carbonylation of 1,1-dichloro-1-alkenes, ?-arylation of carboxylic esters, and conversion of aryl and vinyl triflates to aryl and vinyl halides. It is also involved in the synthesis of azepane. Tris DBA is also a novel inhibitor of N-myristoyltransferase-1 with significant antitumor activity.
| | Uses | Tris(dibenzylideneacetone)dipalladium is used in the preparation of semiconducting polymers processed from nonchlorinated solvents into high performance thin film transistors. Also used in the synthesis of polymer bulk-heterojunction solar sells as a semiconductor. | | Reactions | 1. Catalyst precursor for conversion of aryl chlorides, triflates, and nonaflates to nitroaromatics.
2. Catalyst for the synthesis of epoxides.
3. Catalytic asymmetric allylic and homoallylic diamination of terminal olefins.
4. Site-selective benzylic sp3 palladium-catalyzed direct arylation.
5. Palladium-catalyzed one-pot synthesis of tricyclic indolines.
6. Active catalyst for the Suzuki-Miyaura coupling of 2-pyridyl nucleophiles.
7. Catalyst in combination with BINAP for the asymmetric Heck Arylation of olefins.
8. Precursor for palladium-catalyzed carbon-nitrigen bond formation.
9. Catalyst for α-arylation of ketones,
10. Cross-coupling of aryl halides with aryl boronic acids.
| | References |
- https://en.wikipedia.org/wiki/Tris(dibenzylideneacetone)dipalladium(0)
- http://www.sigmaaldrich.com
- https://www.alfa.com
- https://pubchem.ncbi.nlm.nih.gov
- S. S. Bhandarkar, J. Bromberg, C. Carrillo, P. Selvakumar, R. K. Sharma, B. N. Perry, B. Govindarajan, L. Fried, A. Sohn, K. Reddy and J. L. Arbiser, Tris (Dibenzylideneacetone) Dipalladium, a N-Myristoyltransferase-1 Inhibitor, Is Effective against Melanoma Growth In vitro and In vivo, Clinical Cancer Research, 2008, vol. 18, 5743-5748
| | Chemical Properties | dark purple solid | | Uses | suzuki reaction | | Uses | A cycloaddition catalyst. | | Uses | Tris(dibenzylideneacetone)dipalladium is used in the preparation of semiconducting polymers processed from nonchlorinated solvents into high performance thin film transistors. It is also used as a semiconductor in the synthesis of polymer bulk-heterojunction solar cells. | | Preparation | First reported in 1970,Tris(dibenzylideneacetone)dipalladium(0) is prepared from dibenzylideneacetone and sodium tetrachloropalladate. Because it is commonly recrystallized from chloroform, the complex is often supplied as the adduct [Pd2(dba)3·CHCl3].The purity of samples can be variable. | | General Description | Tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3) participates in the synthesis of azepane. Crystal structure of Pd2(dba)3 has been determined by three-dimensional X-ray data. Crystals of Pd2(dba)3 are reported to crystalize in triclinic system. It is widely used Pd(0) source in Pd-mediated transformations. | | Structure and conformation | In [Pd2(dba)3], the pair of Pd atoms are separated by 320 pm but are tied together by dba units.The Pd(0) centres are bound to the alkene parts of the dba ligands. |
| | Tris(dibenzylideneacetone)dipalladium Preparation Products And Raw materials |
|