|
| | Butyraldehyde oxime Basic information |
| | Butyraldehyde oxime Chemical Properties |
| Melting point | -29.5°C | | Boiling point | 152-154 °C | | density | 0.923 | | refractive index | 1.4367 | | Fp | 58 °C | | storage temp. | Refrigerator, under inert atmosphere | | solubility | Chloroform, Methanol (Slightly) | | form | Oil | | pka | 11.03±0.11(Predicted) | | color | Colourless | | Water Solubility | <0.1 g/100 mL at 20 ºC | | BRN | 1698799 | | Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, powdered metals, acids. | | CAS DataBase Reference | 110-69-0(CAS DataBase Reference) | | NIST Chemistry Reference | Butanal, oxime(110-69-0) | | EPA Substance Registry System | Butanal oxime (110-69-0) |
| Hazard Codes | T | | Risk Statements | 36-24-22 | | Safety Statements | 45-36-23 | | RIDADR | 2840 | | RTECS | ES3500000 | | TSCA | Yes | | HS Code | 2928.00.5000 | | HazardClass | 3 | | PackingGroup | III | | Toxicity | mouse,LD50,intraperitoneal,200mg/kg (200mg/kg),National Technical Information Service. Vol. AD277-689, |
| | Butyraldehyde oxime Usage And Synthesis |
| Chemical Properties | colourless liquid | | Uses | Butyraldoxime can be used to create an anti-counterfeiting ink formulation. | | Preparation | To a slowly stirred mixture of 73 gm (1 mole) of butylamine and 109 gm of a 12.2% aqueous solution of sodium tungstate is added (1.75 hr) with cooling to maintain a temperature of 15°C, 220 gm of 28% hydrogen peroxide. During this reaction 130 ml of ethanol is added portionwise to clarify the emulsion which tends to form. The green solution is stirred at 15°C for an additional hour after the addition has been completed. The reaction mixture is cooled, neutralized, and freed of the ethanol under reduced pressure. The solution is then saturated with sodium chloride and the oily product is separated and distilled to afford 50 gm (58%), b.p. 152°C.
Evidently hydroxylamines may also be oxidized with hydrogen peroxide in the presence of sodium tungstate, as mentioned above. In a recent report, phenylglyoxime was prepared by a ferric chloride oxidation of the correspondingly hydroxylamine oxime.
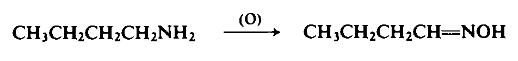
| | Definition | ChEBI: An aldoxime derived from n-butanal. | | General Description | A liquid. Flash point 140°F. Slightly less dense than water. Vapors heavier than air. Used to make plastics and rubber. | | Air & Water Reactions | Flammable. Slightly soluble in water. | | Reactivity Profile | Butyraldehyde oxime is highly explosive during vacuum distillation. Butyraldehyde oxime is incompatible with oxidizing materials. Butyraldehyde oxime is also incompatible with metallic impurities. Butyraldehyde oxime may react with strong acids. | | Health Hazard | May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. | | Fire Hazard | HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. | | Safety Profile | A poison by
intraperitoneal route. Mutation datareported. Flammable liquid when exposed to
heat or flame. To fight fire, use alcohol
foam, dry chemical. Highly explosive. Can
explode during vacuum disdlation.
Incompatible with oxidzing materials,
metallic impurities. When heated to
decomposition it emits toxic fumes of NOx.
See also ALDEHYDES. |
| | Butyraldehyde oxime Preparation Products And Raw materials |
|