|
| | Bromotrimethylsilane Basic information | | Uses |
| Product Name: | Bromotrimethylsilane | | Synonyms: | TMBS, Trimethylbromosilane, Trimethylsilyl bromide;Bromotrimethylsilane Trimethylsilylbromide;Bromotrimethylsilane,TMBS, Trimethylbromosilane, Trimethylsilyl bromide;Bromotrimethylsilane, AcroSeal, 98%;BroMotriMethylsilane, Stab. With Copper Powder or Silver Wire;Bromotrimethylsilane,98%;Trimethylsilyl bromide, TMBS;BroMotriMethylsilane, 98% 100GR | | CAS: | 2857-97-8 | | MF: | C3H9BrSi | | MW: | 153.09 | | EINECS: | 220-672-0 | | Product Categories: | Alkyl Silanes;Blocking Agents;Pharmaceutical Intermediates;Synthetic Organic Chemistry;Protective Agents;Silylating Agents;Si-X (F, Br, I) Compounds;Si (Classes of Silicon Compounds);Silicon Compounds (for Synthesis);2857-97-8 | | Mol File: | 2857-97-8.mol |  |
| | Bromotrimethylsilane Chemical Properties |
| Melting point | -43 °C | | Boiling point | 79 °C(lit.) | | density | 1.16 g/mL at 25 °C(lit.) | | refractive index | n20/D 1.424(lit.) | | Fp | 90 °F | | storage temp. | Store below +30°C. | | solubility | DECOMPOSES | | form | Liquid | | color | Clear colorless to yellow | | Specific Gravity | 1.1725 | | Water Solubility | DECOMPOSES | | Sensitive | Moisture Sensitive | | Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents | | BRN | 1731135 | | Stability: | Stable. Flammable. Incompatible with strong acids, strong oxidizing agents. May decompose on exposure to water or moist air. | | CAS DataBase Reference | 2857-97-8(CAS DataBase Reference) | | NIST Chemistry Reference | Bromotrimethylsilane(2857-97-8) | | EPA Substance Registry System | Silane, bromotrimethyl- (2857-97-8) |
| | Bromotrimethylsilane Usage And Synthesis |
| Uses | Bromotrimethylsilane can protect or deprotect functional groups selectively, act as silane blocking agent, which is widely used in the syntheses of drugs.
| | Chemical Properties | Clear yellow liquid | | Physical properties | bp 79°C; d 1.188 g cm?3; n20
D 1.4240; fp 32°C. | | Uses | Bromotrimethylsilane is a mild and selective reagent for cleavage of lactones, epoxides,
acetals, phosphonate esters and certain ethers; effective reagent
for formation of silyl enol ethers; can function as brominating
agent.
Amines react with TMS-Br to form
isolable adducts, which react readily with ketones to form enamines
under mild conditions (eq 15).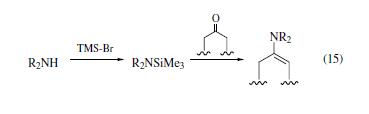 | | Uses | Adefovir intermediate | | Uses | Powerful silylating agent | | Preparation | although many methods are reported,
only a few are provided here: chlorotrimethylsilane undergoes
halogen exchange with either magnesium bromide in Et2O
or sodium bromide in MeCN, which allows in situ reagent
formation (eq 1); alternatively, hexamethyldisilane reacts with
bromine in benzene solution or neat, to afford only TMS-Br
with no byproducts (eq 2).4 TMS-Br may also be generated
by reaction of hexamethyldisiloxane and aluminum bromide
(eq 3).However, it should be noted that the reactivity of in
situ generated reagent appears to depend upon the method of
preparation. 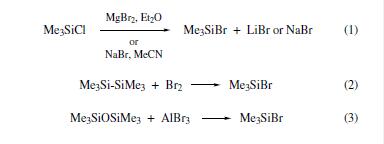
| | General Description | Bromotrimethylsilane is used with Cl3 to catalyze the direct allylation of a variety of alcohols with allyltrimethylsilane. | | Purification Methods | Purify it by repeated fractional distillation and store it in sealed ampoules in the dark. [McCusker & Reilly J Am Chem Soc 75 1583 1953.] Also fractionate it through a 15-plate column (0.8 x 32cm packed with 1/16in single turn helices of Pt-Ir wire). [Gilliam et al. J Am Chem Soc 68 1161 1946, Pray et al. J Am Chem Soc 70 433 1948, Beilstein 4 IV 4008.] |
| | Bromotrimethylsilane Preparation Products And Raw materials |
|