|
| | Plerixafor 8HCl (AMD3100 8HCl) Basic information |
| Product Name: | Plerixafor 8HCl (AMD3100 8HCl) | | Synonyms: | SID791;AMD3100;AMD3100 OCTAHYDROCHLORIDE;BISCYCLAM;JM3100;AMD3100 OCTAHYDROCHLORIDE HYDRATE ANHYDROUS;amd3100 octahydrochloride hydrate;1,1'-[1,4-Phenylenebis-(methylene)]-bis-(1,4,8,11-tetraazacyclotetradecane) octahydrochloride, min. 95 % (NMR) | | CAS: | 155148-31-5 | | MF: | C28H54N8 | | MW: | 502.78 | | EINECS: | 828-184-5 | | Product Categories: | Inhibitors | | Mol File: | 155148-31-5.mol |  |
| | Plerixafor 8HCl (AMD3100 8HCl) Chemical Properties |
| Melting point | 188 - 193°C (dec.) | | storage temp. | Sealed in dry,Store in freezer, under -20°C | | solubility | H2O: ≥10 mg/mL, clear | | form | solid | | color | White | | Water Solubility | Soluble in water (100mM) | | Stability: | Stable for 1 year from date of purchase as supplied. Solutions in distilled water may be stored at -20°C for up to 2 months. |
| | Plerixafor 8HCl (AMD3100 8HCl) Usage And Synthesis |
| Description | Plerixafor (155148-31-5) is a highly selective CXCR4 antagonist (IC50 = 20-130 nM).1,2 Originally investigated as an HIV replication inhibitor.3 Plerixafor has been investigated for the treatment autoimmune diseases and various cancers.4 Clinically useful for the mobilization of hematopoietic stem cells in the treatment of blood cancers. | | Uses | Plerixafor Hydrochloride (1:8), is an immunostimulant used to mobilize hematopoietic stem cells in cancer patients. It is a hematopoietic stem cell (HSC) mobilizer that inhibits the CXCR4 chemokine receptor and blocks binding of its ligand, stromal cell-derived factor-1-α (SDF-1-α). | | Uses | Plerixafor 8HCl (AMD3100 8HCl) is the hydrochloride of Plerixafor, a chemokine receptor antagonist for CXCR4 and CXCL12-mediated chemotaxis with IC50 of 44 nM and 5.7 nM, respectively - See more at: http://www.selleckchem.com/products/plerixafor-8hcl-db06 | | Uses | A specific CXCR4 antagonist. | | General Description | A symmetrical bicyclam compound that antagonizes CXCL12 (SDF1) binding to CXCR4 (IC50 = 108 and 245 nM using rat and human CXCR4, respectively) and inhibits SDF1-induced Ca2+ flux (by 100% at 100 ng/ml in SUP-T1 and THP-1 cultures) as well as CXCR4-, but not CCR5-, mediated HIV infection (IC50 ≤200 nM), while enhancing the binding of SDF1 to CXCR7 (by ~60% at 1 mM with CXCR7-expressing HEK293T cells) and SDF1-induced β-arrestin recruitment to CXCR7 (EC50 = 6.48 and 11.8 nM, in the presence and absence of 10 μM AMD3100, respectively). Administration AMD3100 via intravenous infusion is also reported to result in hematopoietic stem cell mobilization in humans, dogs, and mice in vivo. Exhibits no inhibitory effects against chemokine-induced signaling via CXCR1/2/3 or CCR1/2/3/4/5/6/7/8/9. | | Biological Activity | Highly selective CXCR4 chemokine receptor antagonist (IC 50 values are 0.02 - 0.13 and > 25 μ M for CXCR4 and all other chemokine receptors respectively). Switches inflammatory responses from Th2 to Th1 type and reduces airway hyperresponsiveness in a mouse model of asthma. Potently inhibits HIV-1 and HIV-2 replication in vitro (EC 50 = 4 - 35 nM) and mobilizes hematopoietic stem cells in vivo . | | Biochem/physiol Actions | Cell permeable: yes | | Clinical Use | Plerixafor hydrochloride, a chemokine CXCR4 (SDF-1) antagonist,
was launched by Genzyme for the treatment of enhancing
mobilization of hematopoietic stem cells for autologous transplantation
in patients with lymphoma and enhancing mobilization of
hematopoietic stem cells for transplantation in patients with multiple
myeloma. Being a CXCR4 antagonist, a specific cellular chemokine
receptor, plerixafor triggers the rapid movement of stem
cells out of the bone marrow and into circulating blood where
the stem cells can be collected for use in stem cell transplants.
Regarding its use for cardiac applications, some clinical data suggests
that the presence of stem cells circulating in the bloodstream
or directly injected into the hearts of patients who have suffered a
heart attack may result in improved cardiac function. | | Synthesis | A concise
one-pot synthesis was achieved by a rather novel nitrogen protecting
strategy. Tetraazacyclotetradecane 88 was protected as its
phosphorotriamide 89 by reaction with POCl3 and Et3N in hot DMF. Without isolation, compound 89 was bis-alkylated
with dibromide 90 in the presence of sodium carbonate in refluxing DMF overnight to give bis-phosphoramide 91. The crude dimeric
bis-phosphoramide was hydrolyzed with 3 M HCl to afford
plerixafor hydrochloride (XV) in 62¨C68% yield. 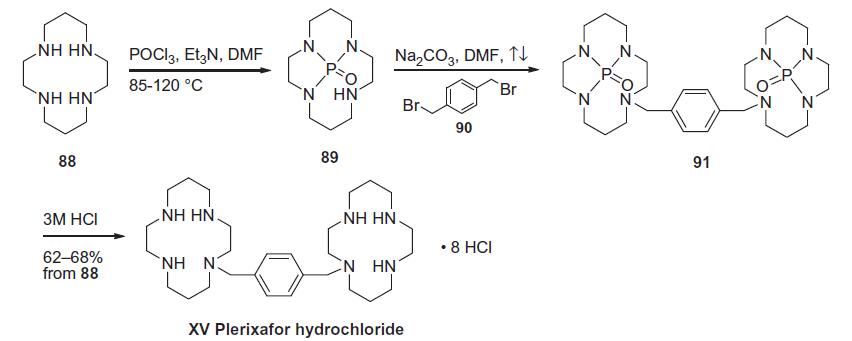
| | storage | -20°C (desiccate) | | References | 1) Donzella, et al. (1998), AMD3100, A Small Molecule Inhibitor of HIV-1 Entry via the CXCR4 Co-Receptor; Nat. Med. 4 72
2) Hatse et al. (2002), Chemokine Receptor Inhibition by AMD3100 Is Strictly Confined to CXCR4; FEBS Lett. 527 255
3) Bridger et al. (1995), Synthesis and Structure-Activity Relationships of Phenylenebis(methylene)-linked Bis-Tetraazamacrocycles That Inhibit HIV Replication. Effects of Macrocyclic Ring Size and Substituents on the aromatic Linker; J. Med. Chem. 38 366
4) Wang et al. (2020), CXCR4 antagonist AMD3100 (plerixafor): from an impurity to a therapeutic agent; Pharmacol. Res. 159 105010 |
| | Plerixafor 8HCl (AMD3100 8HCl) Preparation Products And Raw materials |
|