|
| | (TRIMETHYLSILYL)DIAZOMETHANE Basic information |
| | (TRIMETHYLSILYL)DIAZOMETHANE Chemical Properties |
| Boiling point | 96 °C | | density | 0.773 g/mL at 25 °C | | refractive index | 1.4362 | | Fp | −31 °F | | storage temp. | Refrigerator | | solubility | Sol most organic solvents; insol H2O. | | form | Solution | | color | White | | Water Solubility | Immiscible with water. Miscible with organic solvents. | | Sensitive | Moisture & Light Sensitive | | BRN | 1902903 | | Stability: | Shock Sensitive | | CAS DataBase Reference | 18107-18-1(CAS DataBase Reference) | | EPA Substance Registry System | Silane, (diazomethyl)trimethyl- (18107-18-1) |
| | (TRIMETHYLSILYL)DIAZOMETHANE Usage And Synthesis |
| Chemical Properties | clear yellow solution | | Physical properties | bp 96 °C/775 mmHg; nD/25 1.4362.2 | | Uses | Trimethylsilyldiazomethane is used as one-carbon homologation reagent; stable, safe substitute for diazomethane;participating in the synthesis reactions of insertion reactions;ketenylation reactions;preparation of homoallylic sulfides;pericyclic reactions;[C-N-N] 1,3-dipole for the preparation of azoles. | | Uses | Reactant for preparation of:
• Macrolactam analogs of the natural product macrolide (-)-A26771B with improved metabolic stability and antibacterial activity
• Aigialomycin D analogues as protein kinase inhibitors for cancer treatment
• Unnatural α-amino acid derivatives containing gem-bisphosphonates via Michael addition reaction
• Capped 4-methylumbelliferyl hyaluronan disaccharides and tetrasaccharides as potential hyaluronidase substrates
• Stictamides A-C as matrix metallopeptidase 12 (MMP12) inhibitors with antitumor invasion activity
• Endothelin converting enzyme (ECE) Inhibitors WS 75624A and WS 75624B via a cross-metathesis approach
• Ent-kaurene derivatives as anti-inflammatory agents
• Desmosdumotin C analogs as potent antitumor agents acting via activation of spindle assembly checkpoint
• Imidazolo[2,1-b]benzothiazole derivatives as potential p53 inhibitors | | Uses | (Trimethylsilyl)diazomethane is used as an analyte in the determination of some phenolic organohalogens in human serum by GC-MS. | | Preparation | Trimethylsilyldiazomethane is prepared by the diazo-transfer reaction of
trimethylsilylmethylmagnesium chloride with diphenyl phosphorazidate
(DPPA) (eq 1). 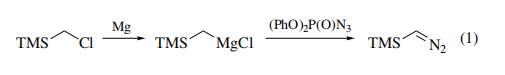
| | General Description | (Trimethylsilyl)diazomethane is considered as a non-explosive substitute to diazomethane for the homologation of carbonyl derivatives, mainly ketones, via reactions such as Tiffeneau-Demjanov rearrangement. |
| | (TRIMETHYLSILYL)DIAZOMETHANE Preparation Products And Raw materials |
|