|
| Product Name: | Thieno[3,2-b]thiophene | | Synonyms: | THIENO[3,2-B]THIOPHENE;1,4-DITHIAPENTALENE;1,4-THIOPHTHENE;1,4-Dithiapentalene
1,4-Thiophthene;BUTTPARK 145\50-51;4-Dithiapentalene 1,4-Thiophthene;Thieno[3,2-b]thiophen;Thieno[3,2-b]thiophene
1,4-Dithiapentalene | | CAS: | 251-41-2 | | MF: | C6H4S2 | | MW: | 140.23 | | EINECS: | | | Product Categories: | Heterocycle-oher series;Electronic Chemicals;Fused Ring Systems;BB16014 | | Mol File: | 251-41-2.mol | ![Thieno[3,2-b]thiophene Structure](CAS/GIF/251-41-2.gif) |
| | Thieno[3,2-b]thiophene Chemical Properties |
| Melting point | 56 °C | | Boiling point | 71 °C / 1mmHg | | density | 1.367±0.06 g/cm3(Predicted) | | Fp | 98℃ | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | soluble in Methanol | | form | powder to crystal | | NIST Chemistry Reference | Thieno[3,2-b]thiophene(251-41-2) | | color | White crystals |
| Hazard Codes | Xn | | Risk Statements | 22-36/37/38 | | Safety Statements | 26-36/37 | | RIDADR | UN 3335 | | WGK Germany | 3 | | Hazard Note | Harmful | | HS Code | 29309090 |
| | Thieno[3,2-b]thiophene Usage And Synthesis |
| General description | Thieno(3,2-b)thiophene (TT) is an electron rich conjugated polymer that has a quinoidal structure with a narrow band gap. It facilitates a strong intermolecular networking.
| | Application | Thieno[3,2-b]thiophene may be used as conjugated side chains in the synthesis of donor-acceptor copolymers. It finds potential applications in the enhancement of power conversion efficiency (PCE) for organic solar cells.
| | Classification | Thiophene, Fused thiophene, Dithiapentalene, Heterocylic aromatics, Five-membered ring, Semiconductor synthesis intermediates, Low band gap polymers OFETs, Organic Photovoltaics, Polymer solar cells
| | preparation | Thieno[3,2-b]thiophene, also known as thienothiophene (TT), is a heterocyclic compound. It belongs to the family of fused thiopehenes and is widely used as an intermediate for the synthesis of small molecules and polymers in the application of organic field-effect transistors (OFETs) and organic photovoltaic devices (OPV).
Fused thiophenes are more electron-rich and more structurally rigid, with extended π-conjugation so they are good candidates for adjusting the band gap of the organic polymer semiconducting materials, i.e. Poly[2,5-bis(3-hexadecylthiophen-2-yl)thieno[3,2-b]thiophene] (also known as PBTTT).
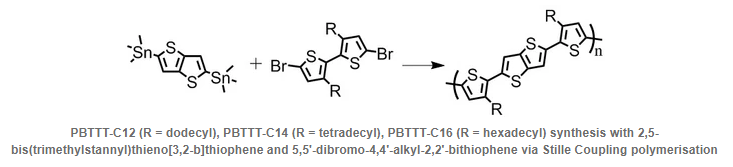
| | Description | Thieno[3,2-b]thiophene, also known as thienothiophene (TT), is a heterocyclic compound. It belongs to the family of fused thiopehenes and is widely used as an intermediate for the synthesis of small molecules and polymers in the application of organic field-effect transistors (OFETs) and organic photovoltaic devices (OPV). | | Chemical Properties | White to yellow Solid | | Uses | TT may be used as conjugated side chains in the synthesis of donor-acceptor copolymers. It finds potential applications in the enhancement of power conversion efficiency (PCE) for organic solar cells. | | General Description | Thieno(3,2-b)thiophene (TT) is an electron rich conjugated polymer that has a quinoidal structure with a narrow band gap. It facilitates a strong intermolecular networking. |
| | Thieno[3,2-b]thiophene Preparation Products And Raw materials |
| Raw materials | Acetic acid,2-(3-thienylthio)--->Thieno[3,2-b]thiophene, 2-(trimethylsilyl)--->3-Bromothiophene-2-carbaldehyde-->METHYL THIENO[3,2-B!THIOPHENE-2-CARBOXYLATE, 97-->Thieno[3,2-b]thiophene-2-methanol-->THIENO[3,2-B]THIOPHENE-2-CARBOXYLIC ACID-->Thieno[3,2-b]thiophene-2-carboxaldehyde-->Thieno[3,2-b]thiophene-2-carboxylic acid ethyl ester | | Preparation Products | 1,2,5,6-Tetrathiocane-->TETRABROMO-THIENO[3,2-B]THIOPHENE-->Thieno[3,2-b]thiophene-2,5-dicarboxaldehyde-->2,5-Dichloro-thieno[3,2-b]thiophene-->4,4,5,5-tetramethyl-2-(thieno[3,2-b]thiophen-2-yl)-1,3,2-dioxaborolane |
|

![Thieno[3,2-b]thiophene Structure](CAS/GIF/251-41-2.gif)

