|
| | Avanafil Basic information |
| Product Name: | Avanafil | | Synonyms: | 4-[[(3-Chloro-4-Methoxyphenyl)Methyl]
aMino]-2-[(2S)-2-(hydroxyMethyl)-1-pyrrolidinyl]-N-(2-pyriMidinylMethyl)-5-pyriMidinecarboxaMide;4-((3-chloro-4-Methoxybenzyl)aMino)-2-(2-(hydroxyMethyl)pyrrolidin-1-yl)-N-(pyriMidin-2-ylMethyl)pyriMidine-5-carboxaMide;Avanafil PDE5 inhibitor;vanafil;(S)-4-((3-Chloro-4-Methoxybenzyl)aMino)-2-(2-(hydroxyMethyl)pyrrolidin-1-yl)-N-(pyriMidin-2-ylMethyl)pyriMidine-5-carboxaMide;Avanafil 4-[(3-Chloro-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1-pyrrolidinyl]-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide;avanfil;(S)-4-((3-Chloro-4-methoxybenzyl)amino)-2-(2-(hydroxymethyl)pyrrolidin-1-yl)-N-(pyrimidin-2-yl | | CAS: | 330784-47-9 | | MF: | C23H26ClN7O3 | | MW: | 483.95 | | EINECS: | 000-000-0 | | Product Categories: | Avanafil;Inhibitor;Aromatics;Intermediates & Fine Chemicals;Pharmaceuticals;API;Chiral Reagents;Heterocycles;Inhibitors;330784-47-9 | | Mol File: | 330784-47-9.mol |  |
| | Avanafil Chemical Properties |
| Melting point | 150-152°C | | density | 1.372 | | storage temp. | Sealed in dry,Store in freezer, under -20°C | | solubility | DMSO (Slightly), Methanol (Slightly, Heated) | | pka | 11.84±0.46(Predicted) | | form | Solid | | color | White to Off-White | | InChIKey | WEAJZXNPAWBCOA-INIZCTEOSA-N | | SMILES | C1(N2CCC[C@H]2CO)=NC=C(C(NCC2=NC=CC=N2)=O)C(NCC2=CC=C(OC)C(Cl)=C2)=N1 | | CAS DataBase Reference | 330784-47-9(CAS DataBase Reference) |
| | Avanafil Usage And Synthesis |
| Description | Avanafil (Zepeed) was approved by the Korean Health Ministry for the
treatment of erectile dysfunction (ED) in August 2011. Avanafil is a
highly selective type 5 phosphodiesterase (PDE5) inhibitor.
Avanafil is reported to be the most selective PDE5
inhibitor on the market. The onset of Tmax and half-life also varies among
the marketed PDE5 inhibitors. Sildenafil has a Tmax at 1 h and a half-life of
3–5 h. Vardenafil is somewhat similar with a Tmax of 0.6 h and a half-life of
4–6 h. Tadalafil has the longest half-life among the marketed drugs with a
half-life of 17 h. Avanafil has a fast onset of action reaching Tmax in 0.6 h
with a half-life of 1.2 h. A synthesis of avanafil (TA-1790) is described
in the patent literature. The main elimination route of avanafil
is through the bile and feces. Avanafil was also found to be reabsorbed
through enterohepatic recirculation. | | Chemical Properties | White Solid | | Originator | Mitsubishi Tanabe Pharma Corporation (Japan) | | Uses | Avanafil is a highly selective PDE5 inhibitor with IC50 of 1 nM. | | Uses | A phosphodiesterase (PDE5) inhibitor, used to treat erectile dysfunction. | | Definition | ChEBI: A monocarboxylic acid amide obtained by formal condensation of the carboxy group of 4-[(3-chloro-4-methoxybenzyl)amino]-2-[(2S)-2-(hydroxymethyl)pyrrolidin-1-yl]pyrimidine-5-carboxylic acid with the amino group of pyrimidin-2-ylmethylamine
Used for treatment of erectile dysfunction. | | Brand name | Zepeed | | Clinical Use | Avanafil was originally discovered at Tanabe Seiyaku (now
Mitsubishi Tanabe). JW Pharmaceutical (previously Choongwae Pharma) and VIVUS have since developed and launched avanafil,
which is an oral PDE5 inhibitor for the treatment of erectile dysfunction
(ED). Although many marketed PDE5 inhibitors (e.g. sildenafil,
vardenafil and tadalafil) are available for the treatment of ED,
many patients are still unable to achieve the desired results and
experience undesired side-effects with these existing medications.
As such, second-generation PDE5 inhibitors with enhanced PDE5
selectivity, shorter systemic half-lives, and improved tolerability
are desired. Developed to meet these criteria, Avanafil exhibited
good oral bioavailability and PDE5 selectivity in both preclinical
studies and clinical trials. Avanafil had a short onset of action
(35 min) and short half-life (1.5 h). | | Synthesis | The synthesis presented
is based on the published patent procedure and is outlined in the
scheme. Commercially available 4-chloro-2-(methylsulfanyl)pyrimidine-
5-carboxylic acid ethyl ester (25) was treated with 3-
chloro-4-methoxybenzylamine (26) and triethylamine at room
temperature to give 4-benzylaminopyrimidine derivative 27 in 96% yield. Sulfide 27 was then oxidized with m-chloroperbenzoic
acid (m-CPBA), followed bytreatment with L-prolinol and triethylamine
to afford ethyl pyrimidinate 28 in 83% yield. This ester
was then saponified with 10% sodium hydroxide to give pyrimidine-
5-carboxylic acid 29 in 80% yield, which then underwent
conventional amide bond formation using 2-(aminomethyl)
pyrimidine (29a), N-(3-dimethylaminopropal)-N0-ethylcarbodiimide
hydrochloride (EDCI) and 1-hydroxybenzotriazole hydrate
(HOBt) to give avanafil (IV) in 83% yield. 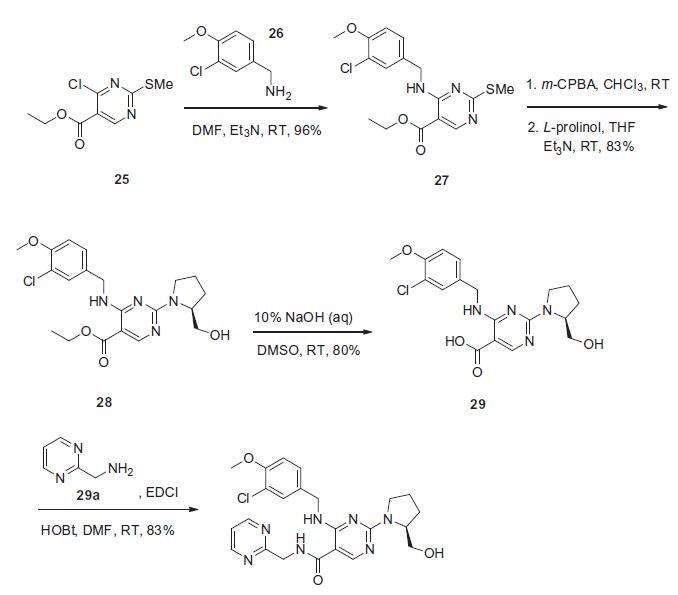
| | Drug interactions | Potentially hazardous interactions with other drugs
Alpha-blockers: enhanced hypotensive effect -
maximum dose 50 mg.
Antibacterials: concentration possibly increased
by clarithromycin and telithromycin - avoid;
concentration increased by erythromycin - reduce
avanafil dose; concentration reduced by rifampicin -
avoid.
Antifungals: concentration increased by ketoconazole
- avoid and fluconazole - reduce avanafil dose;
concentration possibly increased by itraconazole and
voriconazole - avoid.
Antivirals: concentration possibly increased by
atazanavir, indinavir and saquinavir - avoid;
concentration possibly reduced by efavirenz - avoid;
concentration possibly increased by fosamprenavir
- reduce avanafil dose; concentration significantly
increased by ritonavir - avoid.
Aprepitant: concentration possibly increased by
aprepitant - reduce avanafil dose.
Calcium channel blockers: concentration possibly
increased by diltiazem and verapamil - reduce
avanafil dose.
Cobicistat: concentration of avanafil possibly
increased - avoid.
Nicorandil: possibly enhanced hypotensive effect -
avoid.
Nitrates: enhanced hypotensive effect - avoid.
Riociguat: possibly enhanced hypotensive effect -
avoid.
| | Metabolism | Avanafil is metabolised in the liver mainly by the
cytochrome P450 isoenzyme CYP3A4 and to a minor
extent by the CYP2C isoform. Two major metabolites are
produced, one of which is active.
Avanafil is excreted as metabolites mainly in the faeces
(approximately 63%) in the urine (approximately 21%). | | references | [1] kotera j, mochida h, inoue h, noto t, fujishige k, sasaki t, kobayashi t, kojima k, yee s, yamada y, kikkawa k, omori k. avanafil, a potent and highly selective phosphodiesterase-5 inhibitor for erectile dysfunction. j urol. 2012 aug;188(2):668-74.
[2] cui ys, li n, zong ht, yan hl, zhang y. avanafil for male erectile dysfunction: a systematic review and meta-analysis. asian j androl. 2014 may-jun;16(3):472-7. |
| | Avanafil Preparation Products And Raw materials |
|



