|
| | Cyclohexanone oxime Basic information |
| | Cyclohexanone oxime Chemical Properties |
| Melting point | 86-89 °C (lit.) | | Boiling point | 206-210 °C (lit.) | | density | 1.0125 (rough estimate) | | vapor pressure | 1.78Pa at 20℃ | | refractive index | 1.4860 (estimate) | | Fp | 90 °C | | storage temp. | Store below +30°C. | | solubility | 17.7g/l | | pka | 12.42±0.20(Predicted) | | form | Crystalline Powder, Crystals, or Chunks | | color | Off-white to beige-brownish | | Water Solubility | <0.1 g/100 mL at 20 ºC | | BRN | 1616769 | | Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. Reacts violently with fuming sulfuric acid at elevated temperature. | | InChIKey | VEZUQRBDRNJBJY-UHFFFAOYSA-N | | LogP | 1.265 at 25℃ | | CAS DataBase Reference | 100-64-1(CAS DataBase Reference) | | NIST Chemistry Reference | Cyclohexanone, oxime(100-64-1) | | EPA Substance Registry System | Cyclohexanone oxime (100-64-1) |
| | Cyclohexanone oxime Usage And Synthesis |
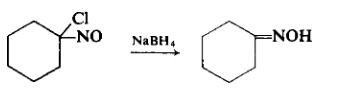
| Chemical Properties | Cyclohexanone oxime, a white crystalline solid, has a molecular weight of 113.16 and a melting point of 90 C. It is soluble in water and ethanol (Lide, 1992). This colorless solid is an important intermediate in the production of nylon 6, a widely used polymer. Cyclohexanone oxime is produced by the condensation of cyclohexanone with hydroxylamine sulfate or hydroxylamine phosphate (Fisher and Cresentini, 1985). | | Uses | Cyclohexanone oxime is used in a wide variety of industrial applications. Primarily, it is used as a captive intermediate in the synthesis of caprolactam, which is polymerized in the production of polycaprolactam (Nylon-6) fibers and plastics (Fisher and Cresentini, 1985; NCI, 1985). The annual U.S. caprolactam production is over 500,000 tons (NCI, 1985). Approximately 90% of the monomer is used to produce fibers for clothing, carpets, home furnishings, and tire cording. The remaining 10% is used to produce nylon resins for food packaging film, extrusion compounds for bristle filaments and wire coatings, and molded plastics for automobiles and appliances (NCI, 1985). Cyclohexanone oxime is also thought to be an intermediate in the oxidative metabolism of sodium cyclamate, an artificial sweetener (Unger and McMahon, 1981). | | Uses | Cyclohexanone Oxime is used as a cathodic inhibitor which inhibits the corrosion of aluminum in hydrochloric acid. | | Preparation | To a cooled, well-stirred solution of 2 gm (0.0115 mole) of 1-chloro-l-nitrosocyclohexane dissolved in a mixture of 20 ml of ethanol and 10 ml of distilled water is added, in small increments, enough sodium borohydride until the color has been discharged. The reaction mixture, which is neutral, is acidified slightly to pH 4. The product is separated by exhaustive extraction with ether. The ether extract is dried over sodium sulfate, filtered, and freed of ether by evaporation. The colorless crystal mass is pressed dry on a clay plate and recrystallized from petroleum ether to afford 0.94 gm (61%), m.p. 89-90°C.
The partial reduction of aliphatic nitro compounds was mentioned as early as the turn of this century. However, only with the commercialization of nitroalkanes did these reactions achieve any real significance. Among the chemical reducing agents, zinc dust and acetic acid have been recommended.
Hydrogenation of nitrocyclohexane on a silver dichromate catalyst has recently been patented. In this procedure, it is said to be important to control the hydrogen take-up to prevent hydrogenation of the oxime to the hydroxylamine. This is accomplished by venting hydrogen off as soon as the theoretical quantity of hydrogen has been used up to convert the nitro compound to the oxime.
Olefinic nitro compounds have been reduced to the saturated oxime with hydrogen and a palladium-on-carbon catalyst. To maximize the yield of oxime, 0.5-1.0 mole of hydrogen chloride per mole of nitroolefin must be present. Since the by-products contain crude ketones also, a posttreatment with hydroxylamine hydrochloride and sodium acetate has been recommended. By this means, 1-nitrocyclooctene has been converted to cyclooctanone oxime [b.p. 63°C (0.08 mm Hg), m.p. 41.7-42.7°C] and 1-nitro-l-octadecene has been converted to stearaldoxime (m.p. 88-89.8°C). Whether this method is confined to 1-olefin derivatives is not clear.
Nitro olefins have also been reduced with zinc dust and acetic acid, to produce oximinoalkanes. The preparation of 5-ethyl-3-nonanone oxime gives the necessary details. To be noted is that the carbon bearing nitro group in the starting material also bears the double bond. Whether this structural feature is essential if the reduction is to stop at the oxime stage may need further elucidation.
| | Synthesis Reference(s) | Organic Syntheses, Coll. Vol. 2, p. 76, 1943
The Journal of Organic Chemistry, 48, p. 2766, 1983 DOI: 10.1021/jo00164a026
Tetrahedron Letters, 28, p. 4557, 1987 DOI: 10.1016/S0040-4039(00)96563-8 | | General Description | Prisms, liquid or light tan crystalline solid. | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | Cyclohexanone oxime reacts violently with fuming sulfuric acid at temperatures > 302° F. . Several explosions or violent decompositions have occurred during distillation of aldooximes, which may be attributable to the formation of peroxides of various types. This is especially the case in the presence of acid, Chem. Eng. News, 1974, 52(35), 3. A nickel catalyzed aldoxime rearrangement to an amide, went out of control after changing the solvent employed, J. Loss Prev., 1993, 6(2), 69. | | Health Hazard | ACUTE/CHRONIC HAZARDS: When heated to decomposition Cyclohexanone oxime emits toxic fumes of nitrogen oxides. | | Fire Hazard | Flash point data for Cyclohexanone oxime are not available; however, Cyclohexanone oxime is probably combustible. | | Flammability and Explosibility | Highlyflammable | | Purification Methods | Crystallise the oxime from water or pet ether (b 60-80o). [Bousquet Org Synth Coll Vol II 313 1943, Beilstein 7 III 32, 7 IV 21.] |
| | Cyclohexanone oxime Preparation Products And Raw materials |
|



