|
| | 1,1-Diethoxybutane Basic information |
| Product Name: | 1,1-Diethoxybutane | | Synonyms: | Butylaldehyde diethyl acetal;n-Butyraldehyde diethyl acetal;BUTYRALDEHYDE DIETHYL ACETAL;BUTRALDEHYDE DIETHYL ACETAL;Butane, 1,1-diethoxy-;butyraldehyde ethyl acetal;BUTYRALDEHYDE DIETHYLACETAL, 97+%;Butanal diethyl acetal | | CAS: | 3658-95-5 | | MF: | C8H18O2 | | MW: | 146.23 | | EINECS: | 222-913-5 | | Product Categories: | | | Mol File: | 3658-95-5.mol |  |
| | 1,1-Diethoxybutane Chemical Properties |
| Hazard Codes | Xi | | Risk Statements | 10-36/38 | | Safety Statements | 26-37/39-16 | | RIDADR | UN 1993C 3 / PGIII | | WGK Germany | 3 | | HS Code | 29329990 |
| | 1,1-Diethoxybutane Usage And Synthesis |
| Chemical Properties | Clear colorless liquid | | Preparation | To a three-necked flask equipped with an air-tight mechanical stirrer, dropping funnel, reflux condenser, and drying tube, are added 3.0 gm (1.25 gm-atom) magnesium turnings, 50 ml of dry ether, and a small crystal of iodine. Then 5 gm of rt-butyl bromide is added dropwise until 171.0 gm (1.25 mole) has been added. The reaction takes about ½ - l h r if the reaction mixture is cooled. The solution is refluxed for ½ hr, cooled to 50°C, and then 148 gm (1.0 mole) of triethyl orthoformate is added dropwise over a ½-hr period. The reaction mixture is refluxed for 16 hr, crushed ice added to decompose the excess Grignard reagent, the ether separated and washed with water. The water layer is added to a separatory funnel containing 200 ml of ether, treated with acetic acid to pH 7.0, shaken, and the ether separated. The latter ether layer is washed with 10% aqueous sodium carbonate, water, and dried. The latter water layer is extracted twice again with ether (200 ml). The combined ether layers are dried over potassium carbonate and fractionated to afford 128 gm (80%), b.p. 143°-144°.
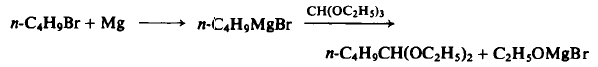
|
| | 1,1-Diethoxybutane Preparation Products And Raw materials |
|