|
| | Chlormezanone Basic information |
| Product Name: | Chlormezanone | | Synonyms: | 2-(4-Chlorophenyl)-3-methyl-1,3-thiazinan-4-one 1,1-dioxide;2-(4-Chlorophenyl)-3-methyl-4-metathiazanone-1,1-dioxide;2-(4-Chlorophenyl)-3-methyl-4-thiazanone 1,1-dioxide;2-(para-chlorophenyl)tetrahydro-3-methyl-4h-1,3-thiazin-4-one,1,1-dioxide;2-(p-chlorophenyl)-3-methyl-1,3-perhydrothiazin-4-on-1,1-dioxide;2-(p-Chlorophenyl)tetrahydro-3-methyl-4H-1,3-thiazin-4-one;2-(4-chlorophenyl)-3-methyl-1,1-dioxo-1,3-thiazinan-4-one;ChlorMezanone (Trancopal) | | CAS: | 80-77-3 | | MF: | C11H12ClNO3S | | MW: | 273.74 | | EINECS: | 201-307-4 | | Product Categories: | Trancopal | | Mol File: | 80-77-3.mol |  |
| | Chlormezanone Chemical Properties |
| Melting point | 114 °C | | Boiling point | 534.5±50.0 °C(Predicted) | | density | 1.2205 (rough estimate) | | refractive index | 1.6300 (estimate) | | storage temp. | Sealed in dry,Room Temperature | | solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) | | form | Solid | | pka | -2.37±0.40(Predicted) | | color | White to Pale Yellow | | Merck | 14,2106 | | NIST Chemistry Reference | Chlormezanone(80-77-3) |
| | Chlormezanone Usage And Synthesis |
| Originator | Trancopal,Winthrop-Breon,US,1958 | | Uses | Skeletal muscle relaxant | | Uses | Chlormezanone improves the emotional state of the patient, relieving moderate anxiety
and stress. However, it has a number of side effects, and because it does not have any
advantage over other anxiolytics, it is rarely used in practice. | | Uses | The use of chlormezanone is associated with large increases in the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. | | Definition | ChEBI: A 1,3-thiazine that is 1,3-thiazinan-4-one S,S-dioxide in which a hydrogen at position 2 is substituted by a 4-chlorophenyl group and the hydrogen attached to the nitrogen is substituted by methyl. A non-benzodiazepine
muscle relaxant, it was used in the management of anxiety and in the treatment of muscle spasms until being discontinued worldwide by its manufacturer in 1996, due to rare but serious cutaneous reactions. | | Manufacturing Process | A solution of 4-chlorobenzaldehyde is reacted with beta-mercaptopropionic
acid and with methylamine. The mixture is refluxed in benzene and water is
removed from an overhead separator. The reaction mixture was cooled,
washed with dilute ammonium hydroxide and water, and the benzene was
removed by distillation in vacuo. The oily residue was taken up in ether from
which it crystallized. The precipitate was recrystallized twice from ether to
yield 2-(4-chlorophenyl)-3-methyl-4-metathiazanone.
A solution of 11.2 g of potassium permanganate in 100 ml of warm water was
added dropwise to a well stirred solution of 10 g of 2-(4-chlorophenyl)-3-
methyl-4-metathiazanone in 50 ml of glacial acetic acid. The temperature was
kept below 30°C with external cooling. An aqueous sodium bisulfite solution
was then added to remove the manganese dioxide. The thick whitish oil which
separated was taken up in chloroform and the extract was washed with water.
Removal of the chloroform by distillation in vacuo yielded an oily residue
which solidified. The solid was recrystallized from isopropyl alcohol to give 5 gof the product, 2-(4-chlorophenyl)-3-methyl-4-metathiazanone-1,1-dioxide,
MP 116.2° to 118.6°C (corr.). | | Brand name | Trancopal (Sanofi
Aventis). | | Therapeutic Function | Tranquilizer | | World Health Organization (WHO) | Chlormezanone is a sedative with antianxiety properties and a
central skeletal muscle relaxant effect. It had already been falling into
obsolescence for several years. | | Biological Activity | Anxiolytic and skeletal muscle relaxant that acts at the benzodiazepine site of GABA A receptors. | | Synthesis | Chlormezanone, 2-(p-chlorophenyl)-tetrahydro-3-methyl-4H-1,3-
tiazin-4-on-1,1-dioxide (5.2.8), is synthesized by joint condensation of mercaptopropionic
acid, methylamine, and 4-chlorobenzaldehyde, evidently through the intermediate stage of
formation of 4-chlorobenzylidenemethylamine, giving the aminothioacetal 2-
(p-chlorophenyl)-tetrahydro-3-methyl-4H-1,3-tiazin-4-one (5.2.7). Oxidation of the sulfur
atom using potassium permanganate gives chlormezanone (5.2.8) [62,63]. 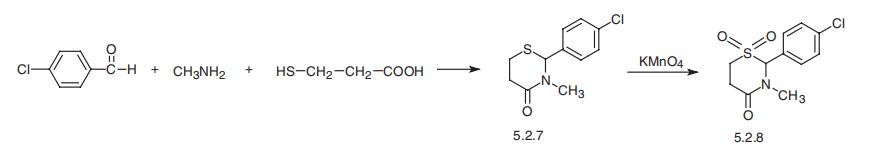
| | storage | Room temperature |
| | Chlormezanone Preparation Products And Raw materials |
|



