|
| | 2,4-DINITROBENZENESULFENYL CHLORIDE Basic information |
| | 2,4-DINITROBENZENESULFENYL CHLORIDE Chemical Properties |
| Hazard Codes | C | | Risk Statements | 34-37 | | Safety Statements | 26-36/37/39-45 | | RIDADR | UN 3261 8/PG 2 | | WGK Germany | 3 | | F | 8-9-19-21 | | HS Code | 2930.90.2900 | | HazardClass | 8 | | PackingGroup | III |
| | 2,4-DINITROBENZENESULFENYL CHLORIDE Usage And Synthesis |
| Uses | 2,4-Dinitrobenzenesulfenyl chloride was used in preparation of chlorine and bromine biphenyls. | | Preparation | CAUTION: This reaction should be carried out in a hood behind a safety shield. The product decomposes explosively if heated above 90-100°C .
To a 500-ml, three-necked flask equipped with a mechanical stirrer, distillation column, and distillation head is added 100 gm (0.25 mole) of dry 2,4-dinitrophenyl disulfide and 250 ml of ethylene chloride. The contents are heated with an oil bath at 125°C until 30-40 ml of the solvent is collected by distillation. The contents are cooled and the distillation head replaced by a gas inlet and exit tube connected to an alkali trap. Then 0.15 gm (0.002 mole) of Merck "Iron by Hydrogen" powder is added as a catalyst [51a-c], and dry chlorine gas is slowly bubbled into the reaction. In a few minutes, the reaction warms up, and the flask is surrounded with a 20°C water bath. The chlorine gas is bubbled in at such a rate that 18 gm (0.25 mole) is added over a 1-1½-hr period. After this time, the suspended disulfide should gradually disappear, and a clear, amber-red solution results. The flow of chlorine gas is stopped, the solution stirred for 1 hr, filtered through diatomaceous earth, the filtrate aspirated to remove excess chlorine, and then 400 ml of anhydrous ether is added. Within ½ hr, most of the product crystallizes by cooling the flask in an ice-salt-water bath for 3-4 hr. The product is filtered, washed with 100 ml of cold ether, and then dried to afford 70 gm (60%) of brilliant yellow needles, m.p. 95-95.5°C.
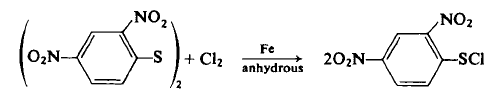
| | Synthesis Reference(s) | Journal of the American Chemical Society, 72, p. 1796, 1950 DOI: 10.1021/ja01160a108 | | General Description | 2,4-Dinitrobenzenesulfenyl chloride undergoes addition reactions with transannularly substituted cycloalkenes and norbornenes. 2,4-Dinitrobenzenesulfenyl chloride can convert allylic alcohols to dienes by 1,4-elimination. | | Purification Methods | Crystallise the sulfenyl chloride from CCl4. [Beilstein 6 II 316.] |
| | 2,4-DINITROBENZENESULFENYL CHLORIDE Preparation Products And Raw materials |
|